

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM20879 C-aryl glucoside, 5::CHEMBL429911::N-ethyl-2,6-dihydroxy-5-[(4-methoxyphenyl)methyl]-3-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]benzamide
SMILES: CCNC(=O)c1c(O)c(Cc2ccc(OC)cc2)cc([C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c1O
InChI Key: InChIKey=ZXQPEYYMUBKGOA-JLMLEOCNSA-N
Data: 3 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20879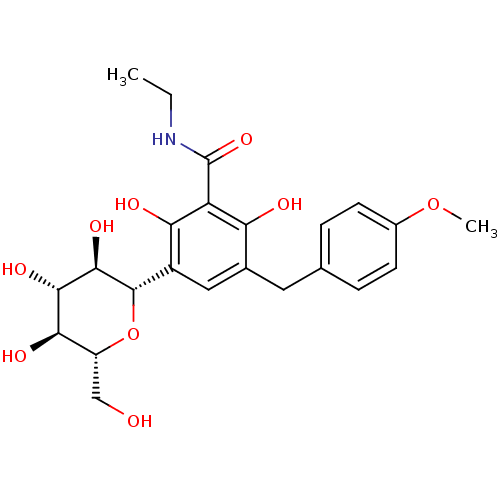 (C-aryl glucoside, 5 | CHEMBL429911 | N-ethyl-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20879 (C-aryl glucoside, 5 | CHEMBL429911 | N-ethyl-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Inhibition of human kidney SGLT2 assessed as renal glucose reabsorption | J Med Chem 52: 1785-94 (2009) Article DOI: 10.1021/jm8013019 BindingDB Entry DOI: 10.7270/Q2F47Q1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (SGLT1) (Homo sapiens (Human)) | BDBM20879 (C-aryl glucoside, 5 | CHEMBL429911 | N-ethyl-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||