

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCNc1nccc(N2CC[C@H](C2)Oc2ccc(cc2)C(C)C(=O)NC2CC2)c1F
InChI Key: InChIKey=SFNHVWJUFOJGLE-OTOKDRCRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 [129-967] (Homo sapiens (Human)) | BDBM212611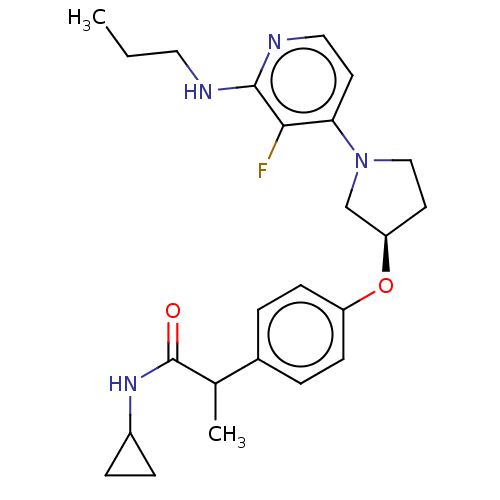 (US9278954, 1.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US9278954 (2016) BindingDB Entry DOI: 10.7270/Q2RB73FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||