

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM21296 1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopropyl)carbonyl]-1H-indol-6-ol::Tetrahydropyranyl-methyl analogue, 17
SMILES: CC1(C)C(C(=O)c2cn(CC3CCOCC3)c3cc(O)ccc23)C1(C)C
InChI Key: InChIKey=YNHUJCRFFWWTHM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21296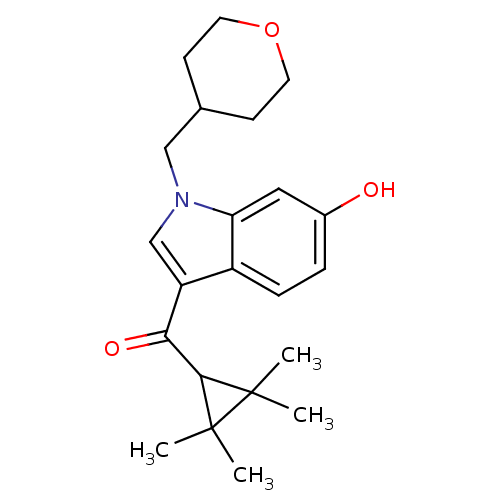 (1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB2 receptor in cell free system | Eur J Med Chem 46: 547-55 (2011) Article DOI: 10.1016/j.ejmech.2010.11.034 BindingDB Entry DOI: 10.7270/Q2CF9QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21296 (1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21296 (1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB1 receptor in cell free system | Eur J Med Chem 46: 547-55 (2011) Article DOI: 10.1016/j.ejmech.2010.11.034 BindingDB Entry DOI: 10.7270/Q2CF9QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21296 (1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | >-7.66 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||