

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM21364 3-[(E)-[(5-bromo-1H-indol-3-yl)methylidene]amino]guanidine::5-bromoindole aminoguanidine, 6
SMILES: NC(N)=NN=Cc1c[nH]c2ccc(Br)cc12
InChI Key: InChIKey=FENBNNFSZUQLQX-UHFFFAOYSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21364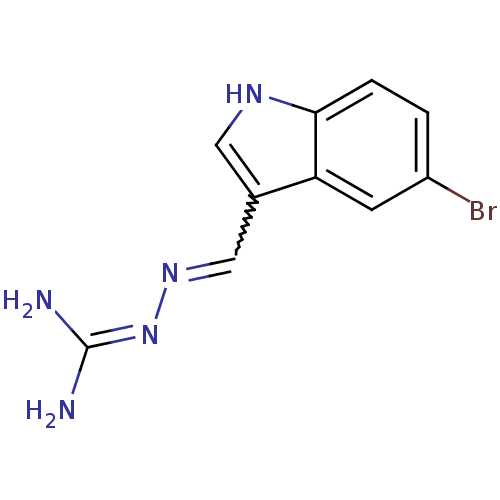 (3-[(E)-[(5-bromo-1H-indol-3-yl)methylidene]amino]g...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description IC50 values for each test compound were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibiti... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||