

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)CC(NC(C)=O)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O
InChI Key: InChIKey=JNRYHIIHOXLMCO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21464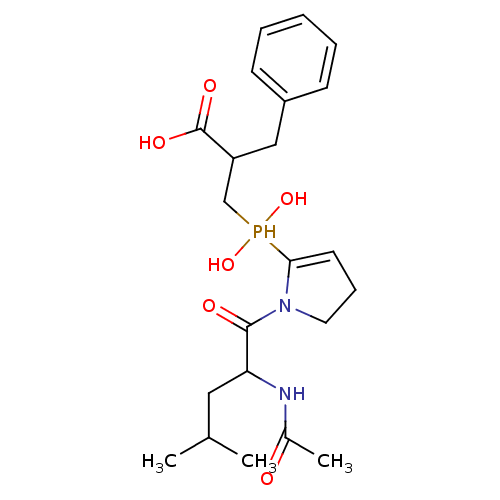 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -13.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21464 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description Inhibitor potencies toward bovine carboxypeptidase A (Sigma-Aldrich Co) were determined in 96-well microplates format. Activity was monitored by meas... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM21464 (2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens | Assay Description Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... | J Med Chem 51: 2216-2226 (2008) Article DOI: 10.1021/jm701275z BindingDB Entry DOI: 10.7270/Q2QF8R4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||