

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM220362 US9290488, B12a
SMILES: C[C@H]1C[C@H]2C[C@@H](C1)C[C@@H](C2)N1[C@H]2CCC[C@@H]1C[C@@H](C2)n1c2ccccc2nc(N2CC(C2)C(O)=O)c1=O
InChI Key: InChIKey=VAXLFHVJPQEDLW-ILEMTEIHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220362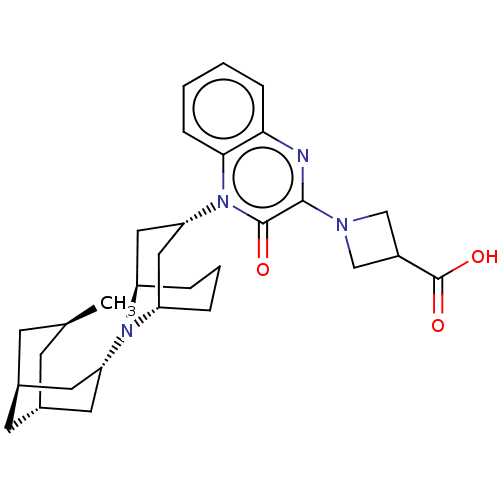 (US9290488, B12a) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.21 | -11.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM220362 (US9290488, B12a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 29.1 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays were conducted using freshly thawed membranes expressing human μ-receptors (Perkin Elmer, Shelton, Conn.). Radioligand ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM220362 (US9290488, B12a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 113 | -9.47 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4-0.8 nM [3H]-U69,593 (NEN; 40 Ci/mmole) with 10-20 μg membrane protein (recombinant kappa opioid rece... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM220362 (US9290488, B12a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.62E+3 | -7.61 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 μg membrane protein (recombinant delta opioid re... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220362 (US9290488, B12a) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8.14 | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description ORL-1 membrane solution was prepared by sequentially adding final concentrations of 0.066 μg/μL ORL-1 membrane protein, 10 μg/mL saponin, 3 ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM220362 (US9290488, B12a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed membranes expressing human μ-receptors. Assay reactions were prepared by se... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM220362 (US9290488, B12a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Kappa opioid receptor membrane solution was prepared by sequentially adding final concentrations of 0.026 μg/μL kappa membrane protein (in-hous... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||