

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM222266 (Z)-4-(2-((E)-4-(2-bromophenyl)-1-morpholino-2-oxobut-3-enylidene)hydrazinyl) benzenesulfonamide (4i)
SMILES: NS(=O)(=O)c1ccc(N\N=C(/N2CCOCC2)C(=O)\C=C\c2ccccc2Br)cc1
InChI Key: InChIKey=IXAXMBLJNOJYEA-RSJLAWTDSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM222266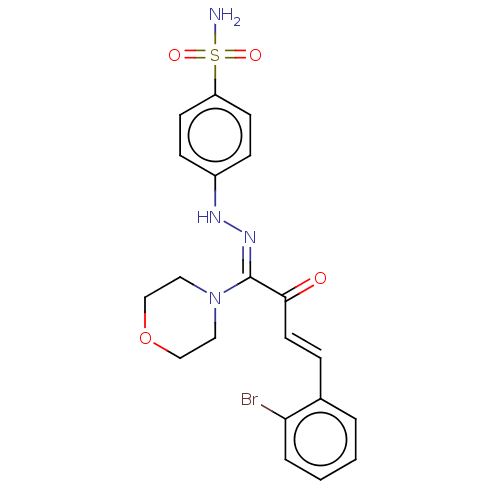 ((Z)-4-(2-((E)-4-(2-bromophenyl)-1-morpholino-2-oxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
COMSATS Institute of Information Technology | Assay Description The ecto-5'-nucleotidase inhibition assay was performed according to our previously reported protocol [Raza et al., Med. Chem., 8:1133-1139]. The com... | Bioorg Chem 70: 229-236 (2017) Article DOI: 10.1016/j.bioorg.2017.01.003 BindingDB Entry DOI: 10.7270/Q2K07334 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal alkaline phosphatase (IAP) (Bos taurus (Cattle)) | BDBM222266 ((Z)-4-(2-((E)-4-(2-bromophenyl)-1-morpholino-2-oxo...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | 9.8 | 37 |
COMSATS Institute of Information Technology | Assay Description Alkaline phosphatase assay was optimized and performed in the same way as previously reported method with slight modifications [Sergienko et al., Nat... | Bioorg Chem 70: 229-236 (2017) Article DOI: 10.1016/j.bioorg.2017.01.003 BindingDB Entry DOI: 10.7270/Q2K07334 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecto-5'-nucleotidase (e5'NT) (Rattus norvegicus (Rat)) | BDBM222266 ((Z)-4-(2-((E)-4-(2-bromophenyl)-1-morpholino-2-oxo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
COMSATS Institute of Information Technology | Assay Description The ecto-5'-nucleotidase inhibition assay was performed according to our previously reported protocol [Raza et al., Med. Chem., 8:1133-1139]. The com... | Bioorg Chem 70: 229-236 (2017) Article DOI: 10.1016/j.bioorg.2017.01.003 BindingDB Entry DOI: 10.7270/Q2K07334 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||