

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM226119 ELTPRESDDRHDSGRD::MT6
SMILES: CC(C)C[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O
InChI Key:
Data: 1 Kd
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA polymerase sigma factor SigB (σB) (Mycobacterium tuberculosis) | BDBM226119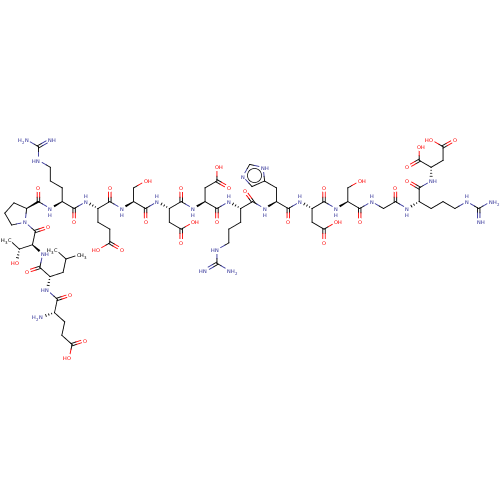 (ELTPRESDDRHDSGRD | MT6) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Science | Assay Description The experiments were conducted using the Octet Red System (Forte bio, MenloPark, CA). Biotinylated peptides were immobilized on kinetics grade Strept... | Biochemistry 56: 2209-2218 (2017) Article DOI: 10.1021/acs.biochem.6b01267 BindingDB Entry DOI: 10.7270/Q2736PR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||