

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22793 1‐phenylindole‐2,3‐dione (Compound 6)::1-phenyl-2,3-dihydro-1H-indole-2,3-dione::Isatin-based compound, 13
SMILES: O=C1N(c2ccccc2C1=O)c1ccccc1
InChI Key: InChIKey=UWCPWBIMRYXUOU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22793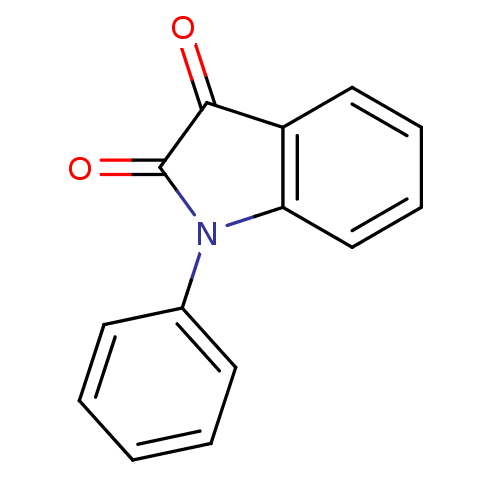 (1‐phenylindole‐2,3‐dione (Compou...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22793 (1‐phenylindole‐2,3‐dione (Compou...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human CE1 using o-NPA as substrate by spectrophotometric assay | J Nat Prod 76: 36-44 (2013) Article DOI: 10.1021/np300628a BindingDB Entry DOI: 10.7270/Q2VX0HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22793 (1‐phenylindole‐2,3‐dione (Compou...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Factor XIIa (Homo sapiens (Human)) | BDBM22793 (1‐phenylindole‐2,3‐dione (Compou...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 950 | -8.21 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylesterase 2 (Homo sapiens (Human)) | BDBM22793 (1‐phenylindole‐2,3‐dione (Compou...) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human iCE using o-NPA as substrate by spectrophotometric assay | J Nat Prod 76: 36-44 (2013) Article DOI: 10.1021/np300628a BindingDB Entry DOI: 10.7270/Q2VX0HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM22793 (1‐phenylindole‐2,3‐dione (Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22793 (1‐phenylindole‐2,3‐dione (Compou...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 50: 1876-85 (2007) Article DOI: 10.1021/jm061471k BindingDB Entry DOI: 10.7270/Q2TX3CNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chorismate mutase (MTB CM) (Mycobacterium tuberculosis H37Rv) | BDBM22793 (1‐phenylindole‐2,3‐dione (Compou...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Birla Institute of Technology & Science-Pilani, Hyderabad Campus, Shameerpet, R.R. District, Hyderabad, Andhra Pradesh, 500078, India | Assay Description The reaction was initiated by the addition of 150pmoles of enzyme to a mixture of inhibitor (40µM, 20 µM, 10 µM, 5 µM, 1 µM ... | Chem Biol Drug Des 83: 498-506 (2014) Article DOI: 10.1111/cbdd.12265 BindingDB Entry DOI: 10.7270/Q2F76B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||