

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22858 1,2-Dione-Based Compound, 15::2-nitro-9,10-dihydrophenanthrene-9,10-dione::CHEMBL433282::NSC23180::US9073941, 904
SMILES: [O-][N+](=O)c1ccc-2c(c1)C(=O)C(=O)c1ccccc-21
InChI Key: InChIKey=KNAXWOBOCVVMST-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22858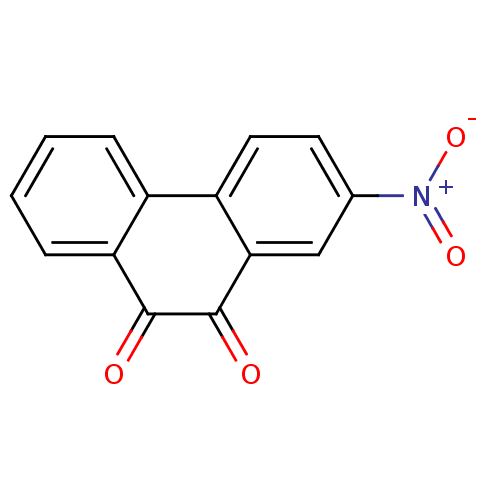 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 5727-34 (2007) Article DOI: 10.1021/jm0706867 BindingDB Entry DOI: 10.7270/Q2Q52MWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Factor XIIa (Homo sapiens (Human)) | BDBM22858 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 107 | -9.50 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
St. Jude Research Hospital | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 50: 5727-34 (2007) Article DOI: 10.1021/jm0706867 BindingDB Entry DOI: 10.7270/Q2Q52MWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22858 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 50: 5727-34 (2007) Article DOI: 10.1021/jm0706867 BindingDB Entry DOI: 10.7270/Q2Q52MWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM22858 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 50: 5727-34 (2007) Article DOI: 10.1021/jm0706867 BindingDB Entry DOI: 10.7270/Q2Q52MWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine Phosphosulfate Reductase (APSR) (Mycobacterium tuberculosis) | BDBM22858 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.81E+3 | n/a | n/a | n/a | 7.5 | 30 |
The Scripps Research Institute | Assay Description Values of Ki were determined from the dependence of the observed rate constant (kobs) on inhibitor concentration. With subsaturing APS, the inhibit... | J Med Chem 51: 6627-30 (2008) Article DOI: 10.1021/jm800571m BindingDB Entry DOI: 10.7270/Q2PK0DFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoamide Dehydrogenase (Lpd) (Mycobacterium tuberculosis) | BDBM22858 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 640 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Academia Sinica US Patent | Assay Description The assay was performed in a manner similar to that described in Bryk et al., Biochemistry (2010) 49:1616-1627 and modified for an online robotics sc... | US Patent US9073941 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate dehydrogenase (PDH) (Mycobacterium tuberculosis) | BDBM22858 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 691 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Academia Sinica US Patent | Assay Description Mtb PDH (Lpd+DlaT+AceE) is provided by Dr. Bryk Ruslana. The assay was performed in a manner similar to that described in Bryk et al., Biochemistry (... | US Patent US9073941 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte common antigen (Homo sapiens (Human)) | BDBM22858 (1,2-Dione-Based Compound, 15 | 2-nitro-9,10-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||