

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM234356 10-Benzyl-3,4,9,10,11,12-hexahydro-2H-pyrido[4′,3′:4,5]thieno[3,2-e]pyrimido[1,2-c]pyrimidin (14)
SMILES: C(N1CCc2c(C1)sc1N=CN3CCCN=C3c21)c1ccccc1
InChI Key: InChIKey=WYEPICZMXLIHIB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM234356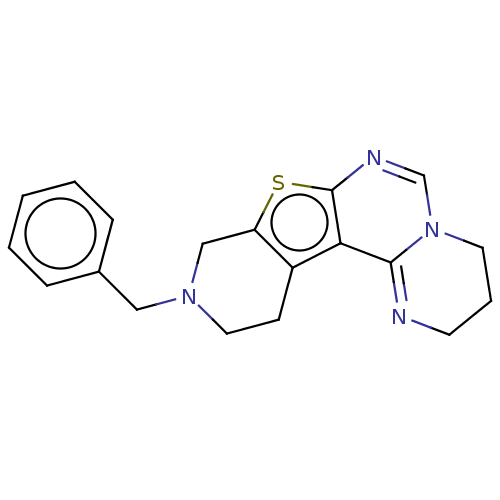 (10-Benzyl-3,4,9,10,11,12-hexahydro-2H-pyrido[4R...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | -7.94 | 1.73E+3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM234356 (10-Benzyl-3,4,9,10,11,12-hexahydro-2H-pyrido[4R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM234356 (10-Benzyl-3,4,9,10,11,12-hexahydro-2H-pyrido[4R...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.25E+4 | n/a | n/a | n/a | n/a | 7.3 | 25 |
University of Bonn | Assay Description Cholinesterase inhibition was assayed spectrophotometrically at 412 nm at 25°C [Pietsch et al., J. Med. Chem., 48:8270-8288; Ellman et al., Bioch... | J Enzyme Inhib Med Chem 26: 350-8 (2011) Article DOI: 10.3109/14756366.2010.504674 BindingDB Entry DOI: 10.7270/Q2959GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||