

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23616 2-benzoylamino benzoic acid deriv., 28j::2-{[3-(4-fluorophenoxy)benzene]amido}benzoic acid
SMILES: OC(=O)c1ccccc1NC(=O)c1cccc(Oc2ccc(F)cc2)c1
InChI Key: InChIKey=HPTXSHNYGZHJLA-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta-Ketoacyl-ACP Synthase III (FabH) (Enterococcus faecalis) | BDBM23616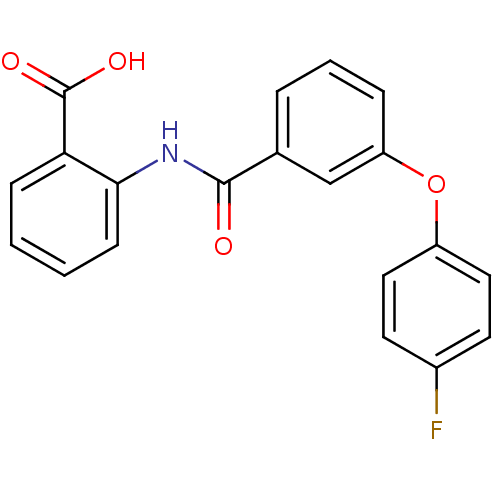 (2-benzoylamino benzoic acid deriv., 28j | 2-{[3-(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shri G.S. Institute of Technology and Science Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis FabH by FabD/FabH coupled assay | Eur J Med Chem 43: 1071-80 (2008) Article DOI: 10.1016/j.ejmech.2007.06.018 BindingDB Entry DOI: 10.7270/Q2GQ700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Ketoacyl-ACP Synthase III (FabH) (Enterococcus faecalis) | BDBM23616 (2-benzoylamino benzoic acid deriv., 28j | 2-{[3-(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quorex Pharmaceuticals Inc. | Assay Description The potency of FabH inhibition (IC50) was determined using [3H]- or [14C]-radiolabeled substrates. This was accomplished at fixed concentrations of a... | J Med Chem 48: 1596-609 (2005) Article DOI: 10.1021/jm049141s BindingDB Entry DOI: 10.7270/Q22B8W9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||