

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23911 3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-yl]-3-oxo-2-[(6-phenoxypyridine-3-)sulfonamido]propyl]benzene-1-carboximidamide::3-amidinophenylalanine deriv., 54
SMILES: [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#6@H](-[#6]-c1cccc(c1)-[#6](-[#7])=[#7])-[#7]S(=O)(=O)c1ccc(-[#8]-c2ccccc2)nc1
InChI Key: InChIKey=OTGQDAVVEHBHSE-VWLOTQADSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor activator/Serine protease hepsin/Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM23911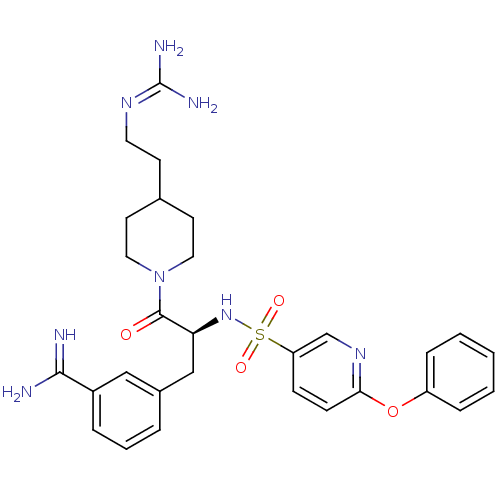 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombin (Bos taurus (Bovine)) | BDBM23911 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM23911 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Factor Xa (fXa) (Bos taurus) | BDBM23911 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM23911 (3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||