

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC(=O)C1CCN(Cc2ccc(Oc3nc4ccccc4s3)cc2)CC1
InChI Key: InChIKey=MPIXODNJTUZDGF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24233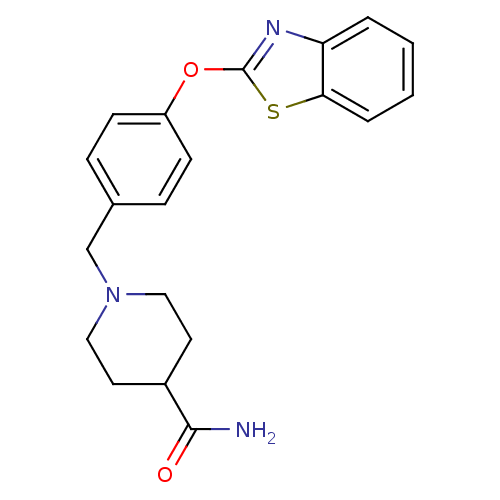 (1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM24233 (1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of dofetilide binding to human ERG by patch clamp assay | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM24233 (1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel. | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||