

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM24440 2-pyridone analogue, 2::JMC521251 Compound 1::N-(3-fluoro-4-{1H-pyrrolo[2,3-b]pyridin-4-yloxy}phenyl)-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide
SMILES: Fc1ccc(cc1)-n1cccc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1=O
InChI Key: InChIKey=OBSFXHDOLBYWRJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24440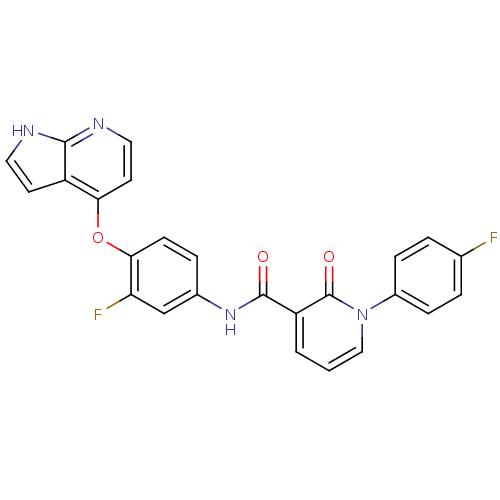 (2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company | Assay Description Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... | J Med Chem 51: 5330-41 (2008) Article DOI: 10.1021/jm800476q BindingDB Entry DOI: 10.7270/Q2K35RZG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24440 (2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company | Assay Description Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate in the presence of test compound. Dose response c... | J Med Chem 52: 1251-4 (2009) Article DOI: 10.1021/jm801586s BindingDB Entry DOI: 10.7270/Q20863MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24440 (2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of human c-MET | Eur J Med Chem 46: 3675-80 (2011) Article DOI: 10.1016/j.ejmech.2011.05.031 BindingDB Entry DOI: 10.7270/Q2891676 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24440 (2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ATP probe from His/GST-tagged c-Met (unknown origin) incubated for 60 mins by HTRF assay | ACS Med Chem Lett 11: 266-271 (2020) Article DOI: 10.1021/acsmedchemlett.9b00065 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase RIPK2 (Homo sapiens (Human)) | BDBM24440 (2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ATP probe from His/GST-tagged RIPK2 (unknown origin) incubated for 60 mins by HTRF assay | ACS Med Chem Lett 11: 266-271 (2020) Article DOI: 10.1021/acsmedchemlett.9b00065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM24440 (2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ATP probe from His/GST-tagged RIPK1 (unknown origin) incubated for 60 mins by HTRF assay | ACS Med Chem Lett 11: 266-271 (2020) Article DOI: 10.1021/acsmedchemlett.9b00065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM24440 (2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ATP probe from full length His/GST-tagged RIPK3 (unknown origin) incubated for 60 mins by HTRF assay | ACS Med Chem Lett 11: 266-271 (2020) Article DOI: 10.1021/acsmedchemlett.9b00065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||