

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM24775 2-methoxy-1,4-dihydronaphthalene-1,4-dione::2-methoxy-1,4-dihydronaphthalene-1,4-dione (5c)::2-methoxy-1,4-naphthoquinone, 2::CHEMBL106562
SMILES: COC1=CC(=O)c2ccccc2C1=O
InChI Key: InChIKey=OBGBGHKYJAOXRR-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM24775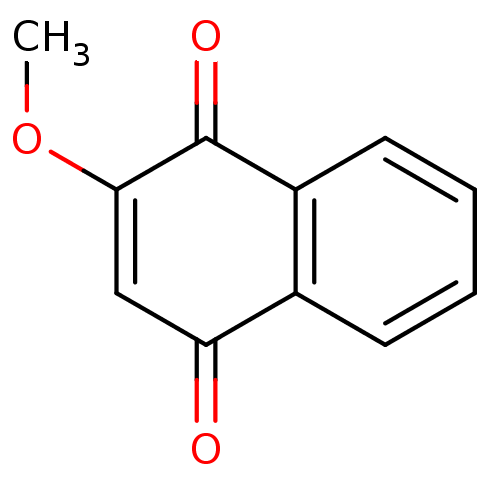 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM24775 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University | Assay Description The protocol for measuring IC50 values for the inhibition of MAO-A and MAO-B has been reported in detail in a recent publication (26). The recombinan... | Chem Biol Drug Des 87: 737-46 (2016) Article DOI: 10.1111/cbdd.12708 BindingDB Entry DOI: 10.7270/Q2MG7N84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase B (MAO-B) (Homo sapiens (Human)) | BDBM24775 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University | Assay Description The protocol for measuring IC50 values for the inhibition of MAO-A and MAO-B has been reported in detail in a recent publication (26). The recombinan... | Chem Biol Drug Des 87: 737-46 (2016) Article DOI: 10.1111/cbdd.12708 BindingDB Entry DOI: 10.7270/Q2MG7N84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM24775 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of SET7 (unknown origin) using biotinylated histone polypeptide as substrate in presence of SAM by AlphaLISA assay | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity phosphatase Cdc25B (Homo sapiens (Human)) | BDBM24775 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University Curated by ChEMBL | Assay Description Inhibition of recombinant Cdc25B (unknown origin) using OMFP as substrate measured every 30 sec for 10 mins by fluorometric assay | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111719 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity phosphatase Cdc25A (Homo sapiens (Human)) | BDBM24775 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University Curated by ChEMBL | Assay Description Inhibition of recombinant Cdc25A (unknown origin) using OMFP as substrate measured every 30 sec for 10 mins by fluorometric assay | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111719 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human rhinovirus A protease (Human rhinovirus B) | BDBM24775 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||