

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM259406 US10457647, Example 5::US9512084, 5
SMILES: Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC1CCN(CC1)C(=O)C=C
InChI Key: InChIKey=SQPBHMYMFCISEC-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259406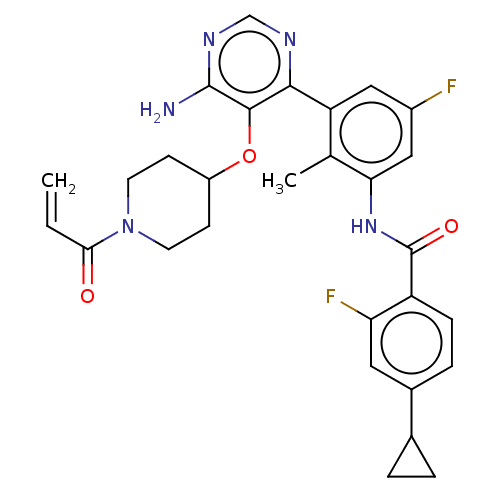 (US10457647, Example 5 | US9512084, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259406 (US10457647, Example 5 | US9512084, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259406 (US10457647, Example 5 | US9512084, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay | J Med Chem 63: 5102-5118 (2020) Article DOI: 10.1021/acs.jmedchem.9b01916 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259406 (US10457647, Example 5 | US9512084, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... | J Med Chem 63: 5102-5118 (2020) Article DOI: 10.1021/acs.jmedchem.9b01916 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259406 (US10457647, Example 5 | US9512084, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BTK in human B cells assessed as reduction in anti-IgM/IL4-stimulated CD69 expression on B cells preincubated for 60 mins followed by a... | J Med Chem 63: 5102-5118 (2020) Article DOI: 10.1021/acs.jmedchem.9b01916 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||