

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26133 (4-methoxyphenyl)boranediol::CHEMBL21427::Phenylboronic Acid, 12
SMILES: COc1ccc(cc1)B(O)O
InChI Key: InChIKey=VOAAEKKFGLPLLU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase AmpC (Escherichia coli) | BDBM26133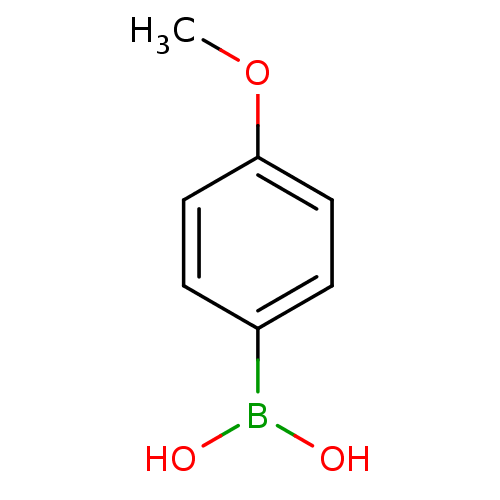 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Inhibitory activity against E. coli AmpC beta-lactamase. | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem Lett 19: 2642-5 (2009) Article DOI: 10.1016/j.bmcl.2009.03.147 BindingDB Entry DOI: 10.7270/Q27D2V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant cytosolic carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem Lett 19: 2642-5 (2009) Article DOI: 10.1016/j.bmcl.2009.03.147 BindingDB Entry DOI: 10.7270/Q27D2V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Carbonic Anhydrase (Candida albicans (Yeast)) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Candida albicans recombinant Carbonic anhydrase preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem Lett 19: 2642-5 (2009) Article DOI: 10.1016/j.bmcl.2009.03.147 BindingDB Entry DOI: 10.7270/Q27D2V4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin (Bacillus licheniformis) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin Carlsberg enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin (Bacillus lentus) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin BL M222C-mutated enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin (Bacillus lentus) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin BL M222S-mutated enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was determined against class A RTEM-1 Beta-lactamase from Escherichia coli | Bioorg Med Chem Lett 4: 1229-1234 (1994) Article DOI: 10.1016/S0960-894X(01)80336-X BindingDB Entry DOI: 10.7270/Q2W9595N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin (Bacillus lentus) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 7.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of Subtilisin BL M222C-mutated enzyme from B. lentus | Bioorg Med Chem Lett 6: 2501-2506 (1996) Article DOI: 10.1016/0960-894X(96)00466-0 BindingDB Entry DOI: 10.7270/Q2X34XFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human C-terminal 6-His-tagged ATXbeta using FS-3 as substrate preincubated for 20 mins followed by substrate addition by fl... | J Med Chem 60: 5209-5215 (2017) Article DOI: 10.1021/acs.jmedchem.6b01224 BindingDB Entry DOI: 10.7270/Q2319ZCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description The endpoint enzymatic assay was developed to quantify human recombinant MGL activity with 2-AG. The formation of arachidonic acid and depletion of ... | J Med Chem 51: 7057-60 (2008) Article DOI: 10.1021/jm801051t BindingDB Entry DOI: 10.7270/Q25D8Q58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM26133 ((4-methoxyphenyl)boranediol | CHEMBL21427 | Phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Oxford | Assay Description [3H]Ethanolamine produced from [3H]AEA hydrolysis was used to calculate FAAH activity and was measured by scintillation counting of the aqueous phase... | J Med Chem 51: 7057-60 (2008) Article DOI: 10.1021/jm801051t BindingDB Entry DOI: 10.7270/Q25D8Q58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||