

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM269371 (S,E)-3-(4-((6-hydroxy- 2-(2-(1- hydroxyethyl)phenyl) benzo[b]thiophen-3- yl)oxy)phenyl)acrylic acid::US10058534, 25
SMILES: C[C@H](O)c1ccccc1-c1sc2cc(O)ccc2c1Oc1ccc(\C=C\C(O)=O)cc1
InChI Key: InChIKey=ISQZMGYHUDJDRI-NRUITVPNSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM269371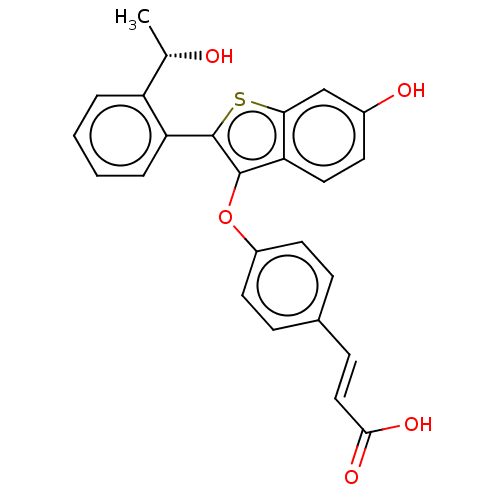 ((S,E)-3-(4-((6-hydroxy- 2-(2-(1- hydroxyethyl)phen...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 179 | n/a | n/a | n/a | n/a | 5.1 | 37 |
Novartis AG US Patent | Assay Description The ER transcription assay is a reporter assay that is based on the ability of ER to induce transcription from a luciferase reporter gene containing ... | US Patent US10058534 (2018) BindingDB Entry DOI: 10.7270/Q2M32XSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM269371 ((S,E)-3-(4-((6-hydroxy- 2-(2-(1- hydroxyethyl)phen...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis | J Med Chem 61: 2837-2864 (2018) Article DOI: 10.1021/acs.jmedchem.7b01682 BindingDB Entry DOI: 10.7270/Q2TB1986 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM269371 ((S,E)-3-(4-((6-hydroxy- 2-(2-(1- hydroxyethyl)phen...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mammalian ribonucleotide reductase | J Med Chem 61: 2837-2864 (2018) Article DOI: 10.1021/acs.jmedchem.7b01682 BindingDB Entry DOI: 10.7270/Q2TB1986 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM269371 ((S,E)-3-(4-((6-hydroxy- 2-(2-(1- hydroxyethyl)phen...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Plate MCF7 cells at 0.3 million cells/mL (100 l/well) in black, clear-bottom 96-well plates (Greiner, catalog number 655090) in DMEM/F12 media (Gibco... | US Patent US10058534 (2018) BindingDB Entry DOI: 10.7270/Q2M32XSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||