

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1cc(Nc2ncnc(n2)-c2ccc(O[C@H]3CCN(C[C@H]3F)C(=O)CO)c(c2)C#N)ccc1N1CCN(CC1)C1COC1
InChI Key: InChIKey=SQZMEALFOPIMFM-NAKRPHOHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM278172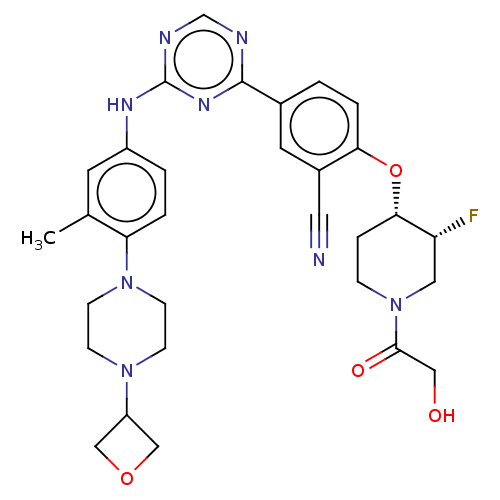 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10040781 (2018) BindingDB Entry DOI: 10.7270/Q22V2J41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM278172 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10040781 (2018) BindingDB Entry DOI: 10.7270/Q22V2J41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM278172 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description Enzymatic activity of hJAK2 was measured using a LANCE homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that monitors... | J Med Chem 50: 6554-69 (2007) BindingDB Entry DOI: 10.7270/Q2Q52RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM278172 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | J Med Chem 50: 6554-69 (2007) BindingDB Entry DOI: 10.7270/Q2Q52RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM278172 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | J Med Chem 50: 6554-69 (2007) BindingDB Entry DOI: 10.7270/Q2Q52RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM278172 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of hJAK2 was measured using a LANCE homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that monitors... | US Patent US10040781 (2018) BindingDB Entry DOI: 10.7270/Q22V2J41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||