

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@@H](Nc1nc(Nc2ccnc(c2)C2(CC2)[N+]#[C-])nc(n1)-n1ccc(n1)C(F)(F)F)C(F)(F)F
InChI Key: InChIKey=OQCGRHOQNOOHFP-SNVBAGLBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM280234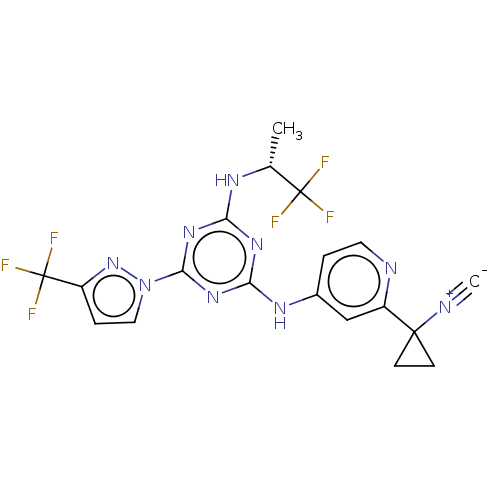 (US10028961, Compound 392 | US10172864, Compound 39...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Agios Pharmaceuticals, Inc. US Patent | Assay Description In the primary reaction, the reduction of α-KG acid to 2-HG is accompanied by a concomitant oxidation of NADPH to NADP. The amount of NADPH rema... | US Patent US10028961 (2018) BindingDB Entry DOI: 10.7270/Q2ZW1NXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM280234 (US10028961, Compound 392 | US10172864, Compound 39...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. US Patent | Assay Description In the primary reaction, the reduction of α-KG acid to 2-HG is accompanied by a concomitant oxidation of NADPH to NADP. The amount of NADPH rema... | US Patent US10172864 (2019) BindingDB Entry DOI: 10.7270/Q2ZK5JQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP], mitochondrial [R140Q] (Homo sapiens (Human)) | BDBM280234 (US10028961, Compound 392 | US10172864, Compound 39...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. US Patent | Assay Description Compounds are assayed for IDH2 R140Q inhibitory activity through a cofactor depletion assay. Compounds are preincubated with enzyme, then the reactio... | US Patent US10172864 (2019) BindingDB Entry DOI: 10.7270/Q2ZK5JQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP], mitochondrial [R140Q] (Homo sapiens (Human)) | BDBM280234 (US10028961, Compound 392 | US10172864, Compound 39...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Agios Pharmaceuticals, Inc. US Patent | Assay Description Compounds are assayed for IDH2 R140Q inhibitory activity through a cofactor depletion assay. Compounds are preincubated with enzyme, then the reactio... | US Patent US10028961 (2018) BindingDB Entry DOI: 10.7270/Q2ZW1NXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||