

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM282900 6-(4-(3-Chlorophenyl)-2-methyl-1H-imidazol-5- yl)quinoline::US10287295, Example 105::US9884868, Example 105
SMILES: Cc1nc(c([nH]1)-c1ccc2ncccc2c1)-c1cccc(Cl)c1
InChI Key: InChIKey=UFWWTBXPJRUJSU-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM282900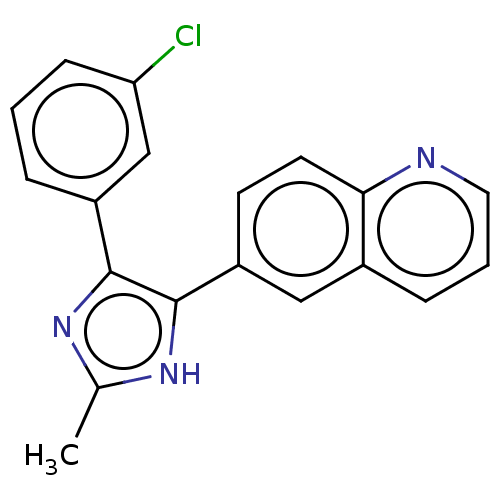 (6-(4-(3-Chlorophenyl)-2-methyl-1H-imidazol-5- yl)q...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rigel Pharmaceuticals, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | US Patent US9884868 (2018) BindingDB Entry DOI: 10.7270/Q20P122M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type II (Homo sapiens (Human)) | BDBM282900 (6-(4-(3-Chlorophenyl)-2-methyl-1H-imidazol-5- yl)q...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin at Madison | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | J Med Chem 51: 7243-52 (2008) BindingDB Entry DOI: 10.7270/Q2B85BFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-betaR1 T204D (Homo sapiens (Human)) | BDBM282900 (6-(4-(3-Chlorophenyl)-2-methyl-1H-imidazol-5- yl)q...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin at Madison | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | J Med Chem 51: 7243-52 (2008) BindingDB Entry DOI: 10.7270/Q2B85BFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type II (Homo sapiens (Human)) | BDBM282900 (6-(4-(3-Chlorophenyl)-2-methyl-1H-imidazol-5- yl)q...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, Inc.; Bristol-Myers Squibb Company US Patent | US Patent US9884868 (2018) BindingDB Entry DOI: 10.7270/Q20P122M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||