

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM283 6-[(8-chloro-4-hydroxy-3-{[4-hydroxy-3-(3-methylbut-2-en-1-yl)benzene]amido}-2-oxo-2H-chromen-7-yl)oxy]-5-hydroxy-3-methoxy-2,2-dimethyloxan-4-yl 5-methyl-1H-pyrrole-2-carboxylate::Clorobiocin::Coumarin-Based, DNA Gyrase Inhibitor::cid_54677920
SMILES: COC1C(OC(=O)c2ccc(C)[nH]2)C(O)C(Oc2ccc3c(O)c(NC(=O)c4ccc(O)c(CC=C(C)C)c4)c(=O)oc3c2Cl)OC1(C)C
InChI Key: InChIKey=FJAQNRBDVKIIKK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hypothetical protein SA1422 (Staphylococcus aureus subsp. aureus N315) | BDBM283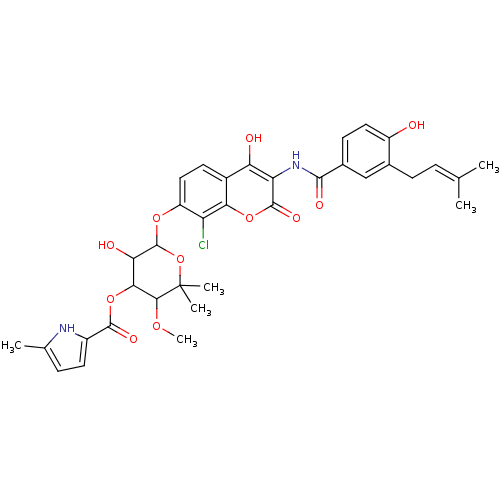 (6-[(8-chloro-4-hydroxy-3-{[4-hydroxy-3-(3-methylbu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PCBioAssay | n/a | n/a | 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2BV7F7H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-galactose 4-epimerase (Trypanosoma brucei) | BDBM283 (6-[(8-chloro-4-hydroxy-3-{[4-hydroxy-3-(3-methylbu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Diego Curated by ChEMBL | Assay Description Inhibition of hexahistidine-tagged Trypanosoma brucei UDP-galactose-4'epimerase expressed in Escherichia coli | J Med Chem 53: 5025-32 (2010) Article DOI: 10.1021/jm100456a BindingDB Entry DOI: 10.7270/Q2M045N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HuR RNA binding protein (Homo sapiens (Human)) | BDBM283 (6-[(8-chloro-4-hydroxy-3-{[4-hydroxy-3-(3-methylbu...) | PDB GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PCBioAssay | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
11762 Curated by PubChem BioAssay | PubChem Bioassay (2015) BindingDB Entry DOI: 10.7270/Q2HQ3XQ7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell (A) | Syringe (B) | Cell Links | Syringe Links | Cell + Syr Links | ΔG° kcal/mole | -TΔS° kcal/mole | ΔH° kcal/mole | log K | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|
| DNA Gyrase (Escherichia coli) | BDBM283 (6-[(8-chloro-4-hydroxy-3-{[4-hydroxy-3-(3-methylbu...) | GoogleScholar PDB | PC cid PC sid PDB | -12.2 | -2.73 | -9.46 | 8.93 | 7.5 | 25 | |
UMR CNRS 6032 | Biochemistry 41: 7217-23 (2002) | |||||||||