

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM283047 6-(1-(2,2-difluoroethyl)-4-(2-hydroxyphenyl)-1H- imidazol-5-yl)imidazo[1,2-b]pyridazine-3-carbonitrile::US10287295, Example 248'::US9884868, Example 248'
SMILES: Oc1ccccc1-c1ncn(CC(F)F)c1-c1ccc2ncc(C#N)n2n1
InChI Key: InChIKey=BYNUJHIHRYVHIS-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM283047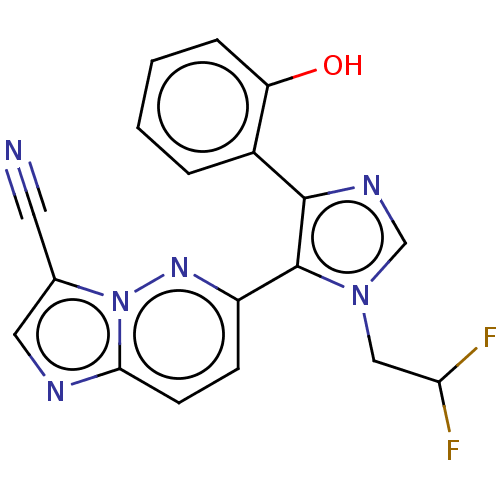 (6-(1-(2,2-difluoroethyl)-4-(2-hydroxyphenyl)-1H- i...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rigel Pharmaceuticals, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | US Patent US9884868 (2018) BindingDB Entry DOI: 10.7270/Q20P122M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type II (Homo sapiens (Human)) | BDBM283047 (6-(1-(2,2-difluoroethyl)-4-(2-hydroxyphenyl)-1H- i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin at Madison | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | J Med Chem 51: 7243-52 (2008) BindingDB Entry DOI: 10.7270/Q2B85BFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-betaR1 T204D (Homo sapiens (Human)) | BDBM283047 (6-(1-(2,2-difluoroethyl)-4-(2-hydroxyphenyl)-1H- i...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin at Madison | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | J Med Chem 51: 7243-52 (2008) BindingDB Entry DOI: 10.7270/Q2B85BFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type II (Homo sapiens (Human)) | BDBM283047 (6-(1-(2,2-difluoroethyl)-4-(2-hydroxyphenyl)-1H- i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel Pharmaceuticals, Inc.; Bristol-Myers Squibb Company US Patent | US Patent US9884868 (2018) BindingDB Entry DOI: 10.7270/Q20P122M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||