

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM286103 US10617673, Example 2a (2S,3R,4'R)::US9517228, Example 2a::US9987253, Example 2a
SMILES: C[C@@H](O)[C@H](N\C(=N/S(=O)(=O)c1ccc(Cl)cc1)N1C[C@H](C(=N1)c1ccc(Cl)cc1)c1ccccc1)C(N)=O
InChI Key: InChIKey=DJDOKEGYGRKFBK-XARZLDAJSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM286103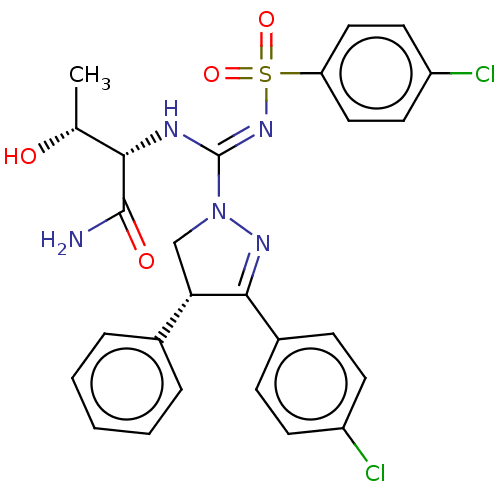 (US10617673, Example 2a (2S,3R,4'R) | US11179370, E...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jenrin Discovery, Inc. US Patent | Assay Description Compound binding to CB1R was assessed in competition displacement assays using [3H]CP-55,940 as the radioligand and crude membranes from mouse brain.... | US Patent US9517228 (2016) BindingDB Entry DOI: 10.7270/Q2M90BPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM286103 (US10617673, Example 2a (2S,3R,4'R) | US11179370, E...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM286103 (US10617673, Example 2a (2S,3R,4'R) | US11179370, E...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jenrin Discovery, LLC US Patent | Assay Description Compound binding to CB1R was assessed in competition displacement assays using [3H]CP-55,940 as the radioligand and crude membranes from mouse brain.... | US Patent US10617673 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM286103 (US10617673, Example 2a (2S,3R,4'R) | US11179370, E...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Newcastle upon Tyne | Assay Description Compound binding to CB1R was assessed in competition displacement assays using [3H]CP-55,940 as the radioligand and crude membranes from mouse brain.... | J Med Chem 49: 6209-21 (2006) BindingDB Entry DOI: 10.7270/Q25T3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||