

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cn1ccnc1CN1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O
InChI Key: InChIKey=QYTBWEJMHPQCLS-GWCFXTLKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginase-1 (Homo sapiens (Human)) | BDBM290395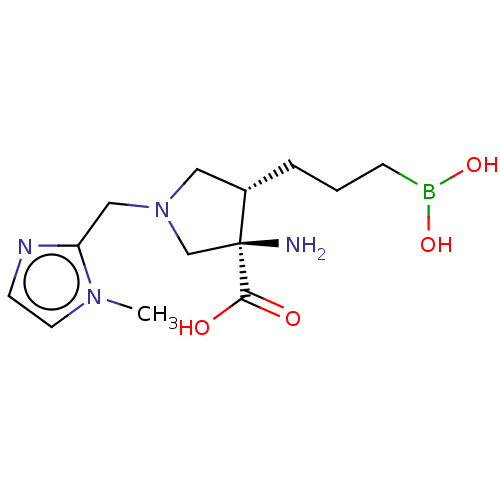 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((1-methyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10098902 (2018) BindingDB Entry DOI: 10.7270/Q2319XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM290395 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10098902 (2018) BindingDB Entry DOI: 10.7270/Q2319XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM290395 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((1-methyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10603330 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | US11389464, Example 31 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM290395 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((1-methyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners Curated by ChEMBL | Assay Description Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... | J Med Chem 62: 8164-8177 (2019) Article DOI: 10.1021/acs.jmedchem.9b00931 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | US11389464, Example 31 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((1-methyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM290395 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10603330 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||