

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: N[C@]1(CN(CC2Cc3c(Cl)cc(Cl)cc3CN2)C[C@@H]1CCCB(O)O)C(O)=O
InChI Key: InChIKey=LCNLEWNKLMUFMQ-PKZGSEHASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginase-1 (Homo sapiens (Human)) | BDBM290423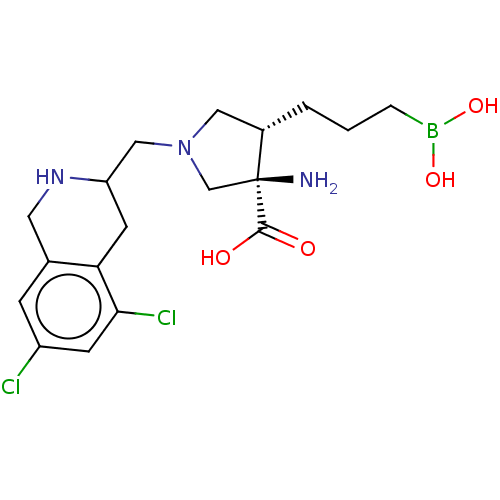 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((5,7-dichlor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10098902 (2018) BindingDB Entry DOI: 10.7270/Q2319XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM290423 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((5,7-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10098902 (2018) BindingDB Entry DOI: 10.7270/Q2319XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM290423 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((5,7-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM290423 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((5,7-dichlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10603330 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM290423 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((5,7-dichlor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM290423 ((3R,4S)-3-amino-4-(3-boronopropyl)-1-((5,7-dichlor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mars, Incorporated US Patent | Assay Description Inhibition of arginase I (ARG I) and arginase II (ARG II) by Formula I or Formula II compounds is followed spectrophotometrically at 530 nm. The comp... | US Patent US10603330 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||