

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM29393 CHEMBL219400::tacrine-dihydropyridine hybrid (tacripyrine), 4
SMILES: CCOC(=O)C1C(c2ccccc2[N+]([O-])=O)c2c(N)c3CCCCc3nc2N=C1C
InChI Key: InChIKey=LLPKFYZXCQQVTR-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29393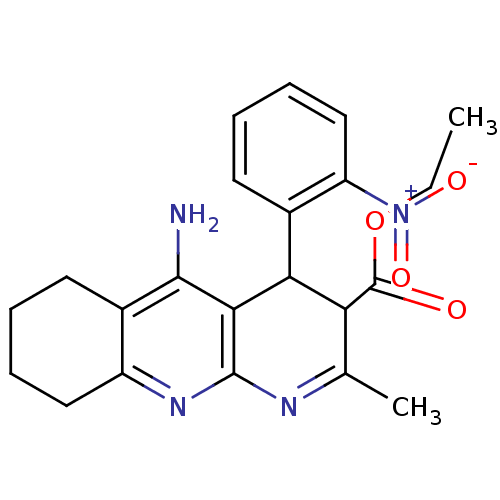 (CHEMBL219400 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 338 | n/a | n/a | n/a | n/a | 7.2 | 22 |
CSIC | Assay Description The inhibitory activity of the compounds towards hAChE was determined following the method of Ellman using AChE from human serum and acetylthiocholin... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM29393 (CHEMBL219400 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
CSIC | Assay Description The inhibitory activity of the compounds towards BuChE was determined following the method of Ellman using BuChE from human serum and butyrylthiochol... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM29393 (CHEMBL219400 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of human serum BuChE | J Med Chem 49: 7607-10 (2006) Article DOI: 10.1021/jm061047j BindingDB Entry DOI: 10.7270/Q26T0M97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM29393 (CHEMBL219400 | tacrine-dihydropyridine hybrid (tac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE | J Med Chem 49: 7607-10 (2006) Article DOI: 10.1021/jm061047j BindingDB Entry DOI: 10.7270/Q26T0M97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29393 (CHEMBL219400 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE | J Med Chem 49: 7607-10 (2006) Article DOI: 10.1021/jm061047j BindingDB Entry DOI: 10.7270/Q26T0M97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29393 (CHEMBL219400 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||