

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM294709 US10112899, Example 187
SMILES: COc1ccc(cc1F)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C
InChI Key: InChIKey=YAMUEPYDRGXRHL-HKBQPEDESA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM294709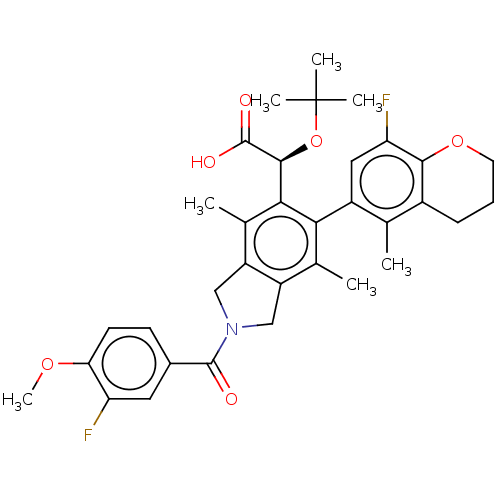 (US10112899, Example 187) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED US Patent | Assay Description Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... | US Patent US10112899 (2018) BindingDB Entry DOI: 10.7270/Q2KP8465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||