

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM301686 US10131692, Compound 18
SMILES: CC(C)CNC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCSCCC(=O)N[C@@H](Cc2ccc(Cl)cc2)C(=O)N[C@@H](Cc2cccs2)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N1
InChI Key: InChIKey=XIJJZASAKZPMAX-MLMVTELGSA-N
Data: 4 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM301686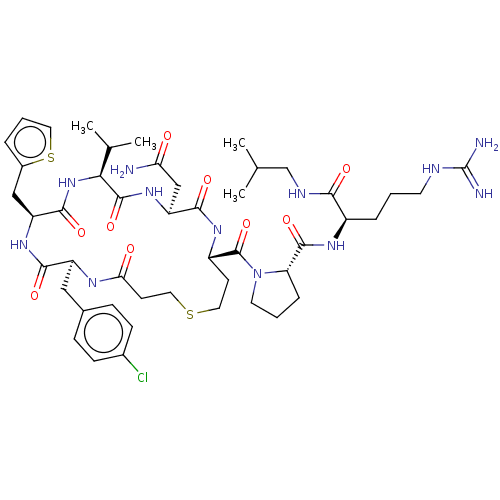 (US10131692, Compound 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Ferring B.V. US Patent | Assay Description Agonist activity of compounds on the human V2 receptor (h V2R) was determined in a transcriptional reporter gene assay by transiently transfecting an... | US Patent US10131692 (2018) BindingDB Entry DOI: 10.7270/Q2WQ05TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM301686 (US10131692, Compound 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc. Curated by ChEMBL | Assay Description Agonist activity at recombinant human V2 receptor expressed in HEK293 cells measured after 5 hrs by cAMP response element driven luciferase reporter ... | J Med Chem 62: 4991-5005 (2019) Article DOI: 10.1021/acs.jmedchem.9b00132 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM301686 (US10131692, Compound 18) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc. Curated by ChEMBL | Assay Description Agonist activity at recombinant human V1B receptor expressed in HEK293 cells measured after 5 hrs by luciferase reporter gene assay | J Med Chem 62: 4991-5005 (2019) Article DOI: 10.1021/acs.jmedchem.9b00132 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM301686 (US10131692, Compound 18) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a |
Ferring B.V. US Patent | Assay Description To determine selectivity, compounds were tested in luciferase-based transcriptional reporter gene assays expressing the human V1b receptor (hV1bR). A... | US Patent US10131692 (2018) BindingDB Entry DOI: 10.7270/Q2WQ05TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||