

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM3021 3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amino)-1H-pyrazolo[3,4-d]pyrimidine::4-(Phenylamino)pyrazolo[3,4-d]pyrimidine deriv. 22::4-N-(3-chlorophenyl)-3-N-[(4-methoxyphenyl)methyl]-1H-pyrazolo[3,4-d]pyrimidine-3,4-diamine
SMILES: COc1ccc(CNc2[nH]nc3ncnc(Nc4cccc(Cl)c4)c23)cc1
InChI Key: InChIKey=KGHAUONMJSXUDW-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3021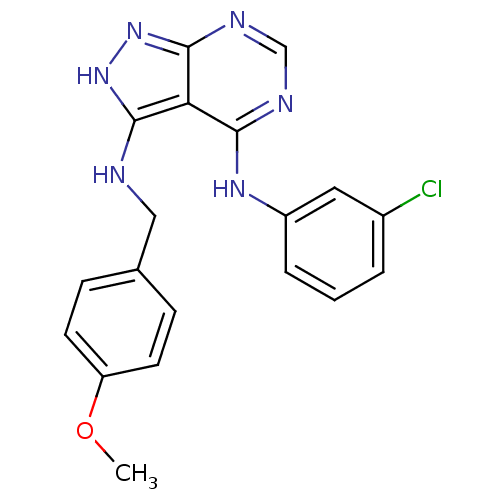 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3021 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 1 (CDK1) (Homo sapiens (Human)) | BDBM3021 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Kinase C, alpha (Bos taurus (bovine)) | BDBM3021 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3021 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||