

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM30406 CHEMBL176058::benzo[1,2,4]thiadiazine-1,1-dioxide, 1
SMILES: CC(C)CCn1c2ccccc2c(O)c(C2=Nc3ccccc3S(=O)(=O)N2)c1=O
InChI Key: InChIKey=MQFPIRFODWNQIO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-structural protein 5B (NS5B) (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM30406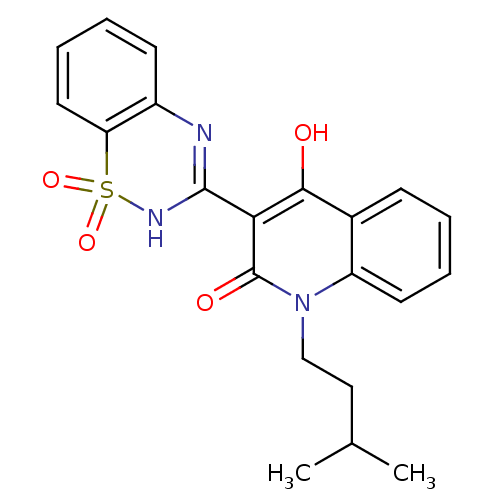 (CHEMBL176058 | benzo[1,2,4]thiadiazine-1,1-dioxide...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 7.5 | 28 |
Anadys Pharmaceuticals | Assay Description Assays were performed in a 96-well streptavidin-coated Flash-Plate using enzyme, RNA substrate, and [alpha-33P]GTP/GTP with inhibitor concentration v... | Bioorg Med Chem Lett 18: 3616-21 (2008) Article DOI: 10.1016/j.bmcl.2008.04.066 BindingDB Entry DOI: 10.7270/Q26D5R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM30406 (CHEMBL176058 | benzo[1,2,4]thiadiazine-1,1-dioxide...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against HCV delta 21 NS5B RNA polymerase by SPA assay | J Med Chem 49: 971-83 (2006) Article DOI: 10.1021/jm050855s BindingDB Entry DOI: 10.7270/Q2N29WJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM30406 (CHEMBL176058 | benzo[1,2,4]thiadiazine-1,1-dioxide...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 417 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HCV RNA replication in Huh7 cells | J Med Chem 49: 971-83 (2006) Article DOI: 10.1021/jm050855s BindingDB Entry DOI: 10.7270/Q2N29WJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM30406 (CHEMBL176058 | benzo[1,2,4]thiadiazine-1,1-dioxide...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus NS5B RNA-dependent RNA polymerase (Hepatitis C virus) | BDBM30406 (CHEMBL176058 | benzo[1,2,4]thiadiazine-1,1-dioxide...) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HCV NS5B RNA dependent RNA polymerase | Bioorg Med Chem Lett 16: 2205-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.034 BindingDB Entry DOI: 10.7270/Q26T0M6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||