

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM305824 (1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrazin-4-yl)-1H-pyrazol-1-yl)-3-(cyanomethyl)cyclobutane-1-carbonitrile::US10144738, Example 23::US10822341, Example 23
SMILES: Cn1cc(c(N)n1)-c1cn2nccc2c(n1)-c1cnn(c1)[C@@]1(CC#N)C[C@@H](C1)C#N
InChI Key: InChIKey=HKTJARAZYORKAF-UWELNFAVSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM305824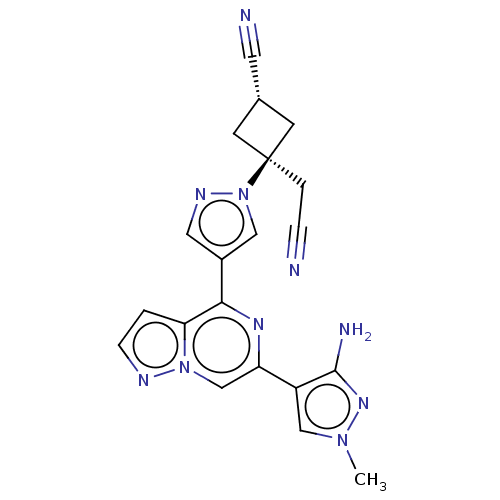 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2XD13RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2XD13RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2XD13RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2XD13RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2SN0C19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10822341 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2SN0C19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2SN0C19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10822341 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10822341 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10822341 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM305824 ((1r,3r)-3-(4-(6-(3-Amino-1-methyl-1H-pyrazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds were added to a 384-well plate. Reaction mixtures contained 10 mM HEPES, pH 7.4, 10 mM MgCl2, 0.01% BSA, 0.0005% Tween 20, 1 mM ATP and 1 &... | US Patent US10144738 (2018) BindingDB Entry DOI: 10.7270/Q2SN0C19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||