

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM30719 (3E)-6-hexyl-3-[(5E)-5-(6-ketocyclohexa-2,4-dien-1-ylidene)-1,3,4-oxadiazolidin-2-ylidene]chromene-2,7-quinone::(3E)-6-hexyl-3-[(5E)-5-(6-oxidanylidenecyclohexa-2,4-dien-1-ylidene)-1,3,4-oxadiazolidin-2-ylidene]chromene-2,7-dione::(3E)-6-hexyl-3-[(5E)-5-(6-oxo-1-cyclohexa-2,4-dienylidene)-1,3,4-oxadiazolidin-2-ylidene]-1-benzopyran-2,7-dione::(3E)-6-hexyl-3-[(5E)-5-(6-oxocyclohexa-2,4-dien-1-ylidene)-1,3,4-oxadiazolidin-2-ylidene]chromene-2,7-dione::MLS000079474::SMR000036273::cid_5389090
SMILES: CCCCCCC1=Cc2c\c(=C3\NN\C(O3)=C3\C=CC=CC3=O)c(=O)oc2=CC1=O
InChI Key: InChIKey=QALVPQWWFNCZOH-LPFJTETCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM30719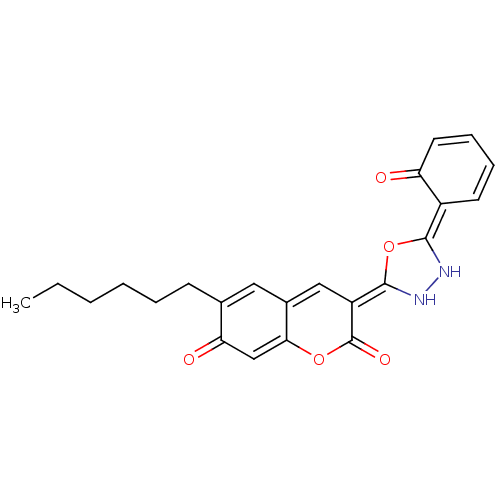 ((3E)-6-hexyl-3-[(5E)-5-(6-ketocyclohexa-2,4-dien-1...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | 9.90E+4 | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description A cell line containing the human 5-HT1a receptor, the promiscuous G-alpha-15 protein (Ga15) as well as the beta-lactamase (BLA) reporter-gene under c... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q21N7ZG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| nucleotide-binding oligomerization domain containing 2 (Homo sapiens (Human)) | BDBM30719 ((3E)-6-hexyl-3-[(5E)-5-(6-ketocyclohexa-2,4-dien-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q23R0R8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM30719 ((3E)-6-hexyl-3-[(5E)-5-(6-ketocyclohexa-2,4-dien-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2JS9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM30719 ((3E)-6-hexyl-3-[(5E)-5-(6-ketocyclohexa-2,4-dien-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q25M6443 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM30719 ((3E)-6-hexyl-3-[(5E)-5-(6-ketocyclohexa-2,4-dien-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2SB447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| nucleotide-binding oligomerization domain containing 1 (Homo sapiens (Human)) | BDBM30719 ((3E)-6-hexyl-3-[(5E)-5-(6-ketocyclohexa-2,4-dien-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | 4.51E+3 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2CN72C0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||