

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31777 thieno[2,3-d]pyrimidine deriv., 2
SMILES: CCc1sc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O
InChI Key: InChIKey=RSFXTHIRRXEJJD-NSHDSACASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM31777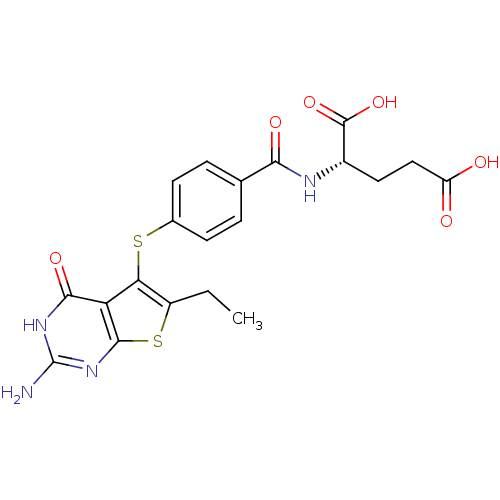 (thieno[2,3-d]pyrimidine deriv., 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate Synthase (TS) (Escherichia coli) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (DHFR) (Toxoplasma gondii) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate Reductase (DHFR) (Escherichia coli) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (DHFR) (Toxoplasma gondii) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||