

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31826 4-aminoquinazoline, 2a::BMC163482 Compound 3::CHEMBL188762
SMILES: CCC(=O)Nc1ccc2ncnc(Nc3cccc(Br)c3)c2c1
InChI Key: InChIKey=WUPUZEMRHDROEO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM31826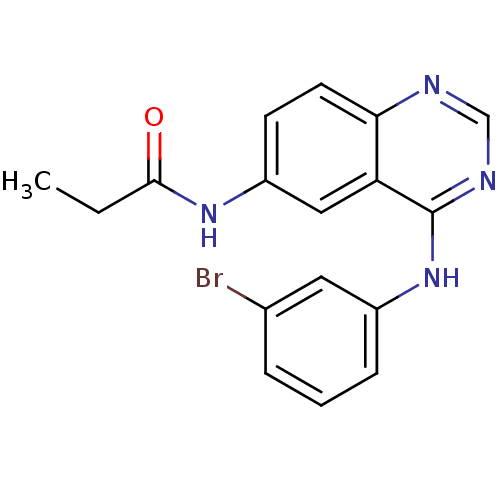 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla... | J Med Chem 52: 3915-26 (2009) Article DOI: 10.1021/jm9002928 BindingDB Entry DOI: 10.7270/Q2VT1QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Src Mutant (T338M) (Gallus gallus (Chicken)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla... | J Med Chem 52: 3915-26 (2009) Article DOI: 10.1021/jm9002928 BindingDB Entry DOI: 10.7270/Q2VT1QD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EGF-R Tyrosine Kinase (Homo sapiens (Human)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description The kinase reaction for EGFR consisted of BSA-supplemented kinase buffer, kinase, peptide, and ATP. For IC50 determinations, 10 different concentrati... | Bioorg Med Chem 16: 3482-8 (2008) Article DOI: 10.1016/j.bmc.2008.02.053 BindingDB Entry DOI: 10.7270/Q2R20ZQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| EGF-R Tyrosine Kinase Mutant (T790M) (Homo sapiens (Human)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description The kinase reaction for EGFR consisted of BSA-supplemented kinase buffer, kinase, peptide, and ATP. For IC50 determinations, 10 different concentrati... | Bioorg Med Chem 16: 3482-8 (2008) Article DOI: 10.1016/j.bmc.2008.02.053 BindingDB Entry DOI: 10.7270/Q2R20ZQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of wild type EGFR by HTRF assay | J Med Chem 53: 2892-901 (2010) Article DOI: 10.1021/jm901877j BindingDB Entry DOI: 10.7270/Q2T43T7N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Src Mutant (S345C/T338M) (Gallus gallus (Chicken)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description IC50 values were determined with the Z lyte assay system (Invitrogen). The reactions were performed in 384-well small volume plates from Greiner (#7... | Bioorg Med Chem 16: 3482-8 (2008) Article DOI: 10.1016/j.bmc.2008.02.053 BindingDB Entry DOI: 10.7270/Q2R20ZQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Ephrin type-B receptor 2 (Homo sapiens (Human)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Protana Inc. Curated by ChEMBL | Assay Description Inhibition of EPH receptor B2 using ELISA | J Med Chem 48: 3221-30 (2005) Article DOI: 10.1021/jm0492204 BindingDB Entry DOI: 10.7270/Q2DF6QRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Gakushuin University Curated by ChEMBL | Assay Description Inhibition of recombinant EGFR by ELISA | Bioorg Med Chem 18: 870-9 (2010) Article DOI: 10.1016/j.bmc.2009.11.035 BindingDB Entry DOI: 10.7270/Q2DF6R97 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Src Mutant (S345C) (Gallus gallus (Chicken)) | BDBM31826 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Chemical Genomics Centre of the Max Planck Society | Assay Description IC50 values were determined with the Z lyte assay system (Invitrogen). The reactions were performed in 384-well small volume plates from Greiner (#7... | Bioorg Med Chem 16: 3482-8 (2008) Article DOI: 10.1016/j.bmc.2008.02.053 BindingDB Entry DOI: 10.7270/Q2R20ZQ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||