

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM325712 US9636336, Example 116::US9636336, Example 128::US9636336, Example 129::[(1S)-2-(3,5-dichloro-1-oxido-pyridin-1-ium-4-yl)-1-(3,4-dimethoxyphenyl)ethyl]5-[[[1-(hydroxymethyl)-2-[[(3R)-1-methylpyrrolidin-3-yl]methoxy]-2-oxo-1-phenyl-ethyl]amino]methyl]thiophene-2-carboxylate
SMILES: COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(CO)(C(=O)OC[C@@H]2CCN(C)C2)c2ccccc2)s1
InChI Key: InChIKey=OQVXVFCVOCOHFS-ZECBPJKWSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325712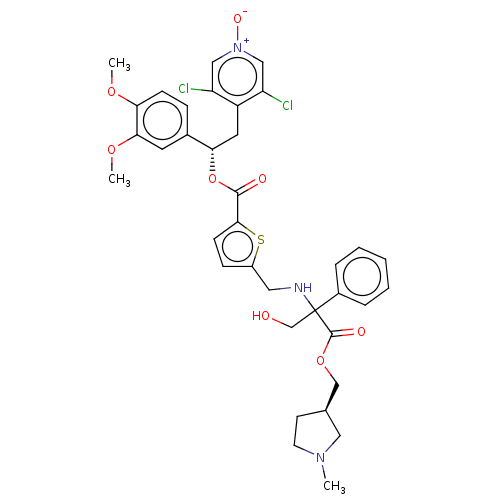 (US9636336, Example 116 | US9636336, Example 128 | ...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 4B2 (Homo sapiens (Human)) | BDBM325712 (US9636336, Example 116 | US9636336, Example 128 | ...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 4B2 (Homo sapiens (Human)) | BDBM325712 (US9636336, Example 116 | US9636336, Example 128 | ...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 4B2 (Homo sapiens (Human)) | BDBM325712 (US9636336, Example 116 | US9636336, Example 128 | ...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325712 (US9636336, Example 116 | US9636336, Example 128 | ...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM325712 (US9636336, Example 116 | US9636336, Example 128 | ...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A. US Patent | Assay Description Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... | US Patent US9636336 (2017) BindingDB Entry DOI: 10.7270/Q2JS9SH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||