

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM338865 US9751888, Compound 55
SMILES: C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1CCc1nnnn1C
InChI Key: InChIKey=LDVDIZLJSHEFSX-LBPRGKRZSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI3-kinase class I (Homo sapiens (Human)) | BDBM338865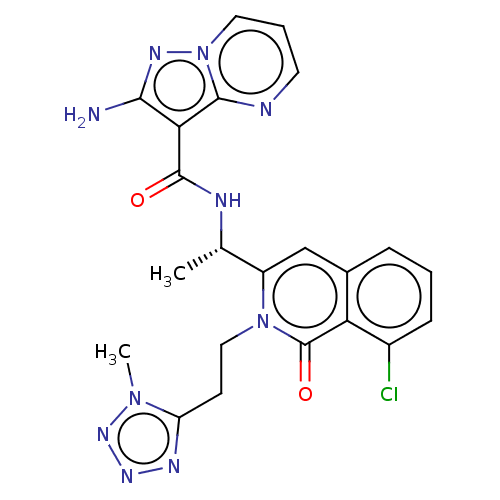 (US9751888, Compound 55) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc. US Patent | Assay Description Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... | US Patent US9751888 (2017) BindingDB Entry DOI: 10.7270/Q2TB190M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM338865 (US9751888, Compound 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc. US Patent | Assay Description Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... | US Patent US9751888 (2017) BindingDB Entry DOI: 10.7270/Q2TB190M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM338865 (US9751888, Compound 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc. US Patent | Assay Description Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... | US Patent US9751888 (2017) BindingDB Entry DOI: 10.7270/Q2TB190M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit beta (Homo sapiens (Human)) | BDBM338865 (US9751888, Compound 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc. US Patent | Assay Description Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... | US Patent US9751888 (2017) BindingDB Entry DOI: 10.7270/Q2TB190M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||