

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM354268 (2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl)-1H-indazol-1- yl)acetyl)-N-(6- bromopyridin-2-yl)-4- fluoropyrrolidine-2- carboxamide::US10287301, Compound 165::US10822352, Comp No. 165::US9796741, cmp No. 165
SMILES: CC(=O)c1nn(CC(=O)N2C[C@H](F)C[C@H]2C(=O)Nc2cccc(Br)n2)c2ccc(cc12)-c1cnc(C)nc1
InChI Key: InChIKey=PIBARDGJJAGJAJ-NQIIRXRSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complement factor D (Homo sapiens (Human)) | BDBM354268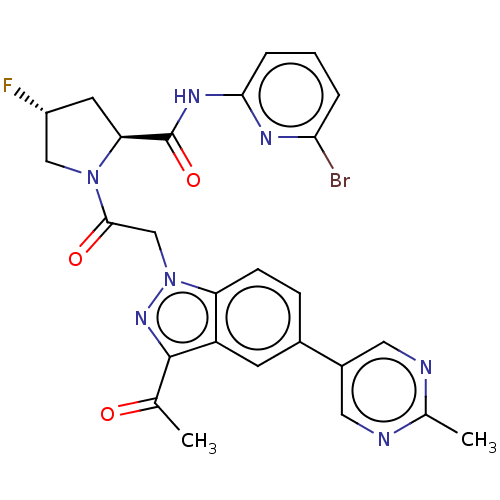 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9796741 (2017) BindingDB Entry DOI: 10.7270/Q2QN68XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description Human Factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration was incubated with test compound at various conc... | J Med Chem 50: 2213-24 (2007) BindingDB Entry DOI: 10.7270/Q22V2JF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Binding affinity to human factor D by SPR analysis | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human Factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration was incubated with test compound at various conc... | US Patent US10822352 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in human serum assessed as decrease in alternative pathway of complement-mediated hemolysis of PNH erythrocytes | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of factor D in human serum assessed as decrease in C3 deposition on PNH erythrocytes | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM354268 ((2S,4R)-1-(2-(3-acetyl- 5-(2-methylpyrimidin- 5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human factor D using C3b as substrate assessed as decrease in formation of factor B cleavage product Ba | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||