 Found 12 hits for monomerid = 36551
Found 12 hits for monomerid = 36551 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM36551
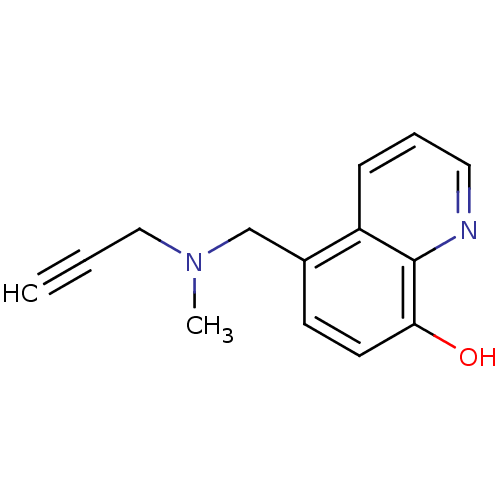
(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Weizmann Institute of Science
| Assay Description
Inhibition of AChE and BChE activity in rat brain homogenates using Ellman's method. |
ACS Chem Biol 5: 603-10 (2010)
Article DOI: 10.1021/cb900264w
BindingDB Entry DOI: 10.7270/Q2959FX1 |
More data for this
Ligand-Target Pair | |
Monoamine Oxidase Type B (MAO-B)
(Rattus norvegicus (rat)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Weizmann Institute of Science
| Assay Description
Inhibition of MAO activity in rat brain homogenates. |
ACS Chem Biol 5: 603-10 (2010)
Article DOI: 10.1021/cb900264w
BindingDB Entry DOI: 10.7270/Q2959FX1 |
More data for this
Ligand-Target Pair | |
Amine oxidase (flavin-containing) A
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... |
US Patent US9034303 (2015)
BindingDB Entry DOI: 10.7270/Q20Z722N |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
MAO-A and MAO-B activity was measured using radioactive substrates. The substrate for MAO-A was 5 HT and for MAO-B was PEA. When measuring the activi... |
US Patent US9034303 (2015)
BindingDB Entry DOI: 10.7270/Q20Z722N |
More data for this
Ligand-Target Pair | |
Monoamine oxidase
(Rattus norvegicus (rat)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A in rat liver homogenate using [14C]-5HT as substrate preincubated for 30 mins followed by substrate addition measured after 20 mi... |
Eur J Med Chem 80: 543-61 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.078
BindingDB Entry DOI: 10.7270/Q2BK1DWK |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MAOB (unknown origin) |
Eur J Med Chem 176: 228-247 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.020 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of BACE-1 (unknown origin) |
Eur J Med Chem 176: 228-247 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.020 |
More data for this
Ligand-Target Pair | |
Amine oxidase (flavin-containing) A
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of MAOA (unknown origin) |
Eur J Med Chem 176: 228-247 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.020 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of MAOB (unknown origin) |
Eur J Med Chem 176: 228-247 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.020 |
More data for this
Ligand-Target Pair | |
Amine oxidase (flavin-containing) A
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of MAOA (unknown origin) |
Eur J Med Chem 176: 228-247 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.020 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of AChE (unknown origin) |
Eur J Med Chem 176: 228-247 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.020 |
More data for this
Ligand-Target Pair | |
Monoamine Oxidase Type B (MAO-B)
(Rattus norvegicus (rat)) | BDBM36551

(Chelator, M30 | US9034303, M30)Show InChI InChI=1S/C14H14N2O/c1-3-9-16(2)10-11-6-7-13(17)14-12(11)5-4-8-15-14/h1,4-8,17H,9-10H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... |
Eur J Med Chem 80: 543-61 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.078
BindingDB Entry DOI: 10.7270/Q2BK1DWK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data