 Found 14 hits for monomerid = 368199
Found 14 hits for monomerid = 368199 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM368199
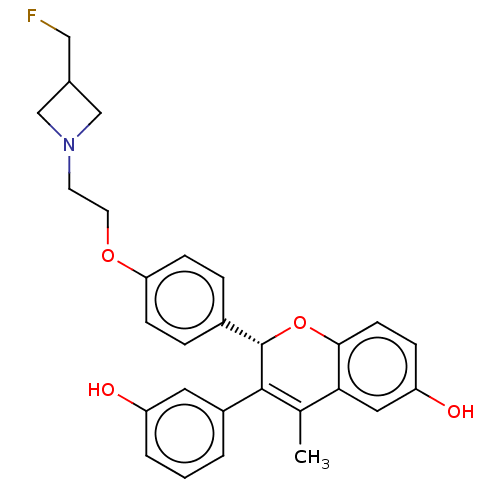
((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
MCF-7 cells were adjusted to a concentration of 40,000 cells per mL in RPMI containing 10% FBS and 20 mM HEPES. 16 microliters of the cell suspension... |
J Med Chem 49: 2868-75 (2006)
BindingDB Entry DOI: 10.7270/Q2PN97Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis |
Bioorg Med Chem Lett 29: 2090-2093 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.013 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of dopamine transporter (unknown origin) |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human T47D cells |
ACS Med Chem Lett 11: 1342-1347 (2020)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as decrease in ERalpha protein level after 4 hrs by InCell Western assay |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 4 hrs by Alexafluor-488 conjugate anti-mouse IgG antibody/Hoechst 33342 staining based imm... |
Bioorg Med Chem Lett 29: 905-911 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.036 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst staining based immunofluorescence imaging analysis |
ACS Med Chem Lett 11: 1342-1347 (2020)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM368199

((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...)Show SMILES CC1=C([C@@H](Oc2ccc(O)cc12)c1ccc(OCCN2CC(CF)C2)cc1)c1cccc(O)c1 |r,t:1| Show InChI InChI=1S/C28H28FNO4/c1-18-25-14-23(32)7-10-26(25)34-28(27(18)21-3-2-4-22(31)13-21)20-5-8-24(9-6-20)33-12-11-30-16-19(15-29)17-30/h2-10,13-14,19,28,31-32H,11-12,15-17H2,1H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 10: 50-55 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00414 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data