

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)NC[C@@H](Nc1ncnc2c(ccnc12)C(N)=O)c1ccc(F)c(c1)C(F)(F)F
InChI Key: InChIKey=ORFQXNMTLHYGKN-OAHLLOKOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM369761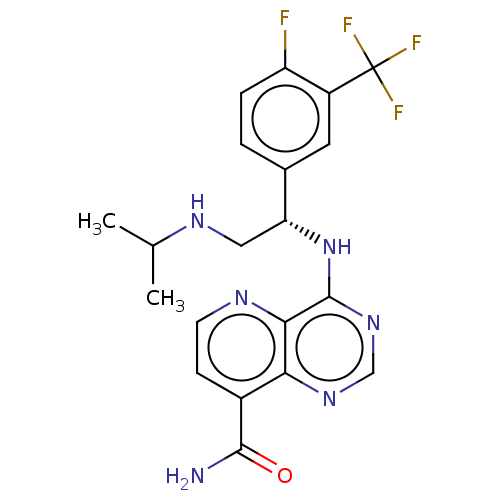 (US10233160, Compound 52 | US9981925, Compound 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description p70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components were then added to the co... | J Med Chem 51: 2216-2226 (2008) BindingDB Entry DOI: 10.7270/Q27H1MWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM369761 (US10233160, Compound 52 | US9981925, Compound 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals | Assay Description In order to measure AKT inhibition in the Caliper Life Sciences LC3000, a TTP Mosquito liquid handling instrument was used to place 125 nl of the app... | Bioorg Med Chem Lett 18: 4181-5 (2008) BindingDB Entry DOI: 10.7270/Q2319Z6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase beta-1 (Homo sapiens (Human)) | BDBM369761 (US10233160, Compound 52 | US9981925, Compound 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <25 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals | Assay Description p70S6K inhibitor compounds were diluted and plated in 96 well plates. A reaction mixture including the following components were then added to the co... | Bioorg Med Chem Lett 18: 4181-5 (2008) BindingDB Entry DOI: 10.7270/Q2319Z6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM369761 (US10233160, Compound 52 | US9981925, Compound 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens | Assay Description In order to measure AKT inhibition in the Caliper Life Sciences LC3000, a TTP Mosquito liquid handling instrument was used to place 125 nl of the app... | J Med Chem 51: 2216-2226 (2008) BindingDB Entry DOI: 10.7270/Q27H1MWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||