

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM371780 N-(6-fluoropyridin-2-yl)-4-(2-(6-methylpyridin-3-yl)-4-(trifluoromethyl)phenyl)chroman-7-sulfonamide::US10239869, Example 206
SMILES: Cc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1cccc(F)n1)C(F)(F)F
InChI Key: InChIKey=BCWBNSSZRLWCHD-OAQYLSRUSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371780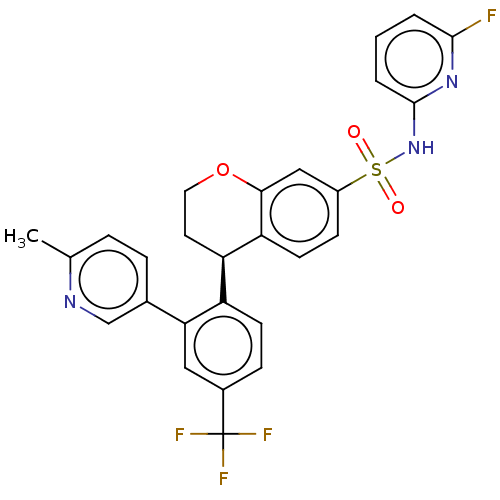 (N-(6-fluoropyridin-2-yl)-4-(2-(6-methylpyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco | Assay Description HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... | J Med Chem 51: 545-52 (2008) BindingDB Entry DOI: 10.7270/Q2NG4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha/beta-1/beta-2 (Homo sapiens (Human)) | BDBM371780 (N-(6-fluoropyridin-2-yl)-4-(2-(6-methylpyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.5 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM371780 (N-(6-fluoropyridin-2-yl)-4-(2-(6-methylpyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... | J Med Chem 63: 6107-6133 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM371780 (N-(6-fluoropyridin-2-yl)-4-(2-(6-methylpyridin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco | Assay Description HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... | J Med Chem 51: 545-52 (2008) BindingDB Entry DOI: 10.7270/Q2NG4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||