

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(=O)N1CCN(CC1)c1ccc(NC(=O)Cc2cnc(c(C)c2)-c2ccnc(F)c2)nc1
InChI Key: InChIKey=AXXNRMISICMFNS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Wnt-3a (Homo sapiens (Human)) | BDBM374998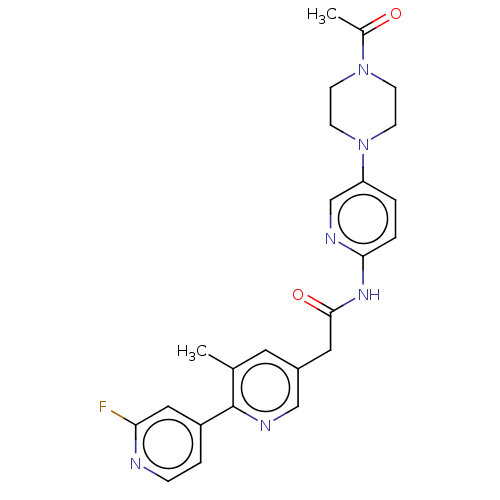 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description Human embryonic kidney 293 cells (obtained from American Type Culture Collection, ATCC, Manassas, Va.) are cultured in DMEM medium (Gibco/Invitrogen,... | J Med Chem 50: 5727-34 (2007) BindingDB Entry DOI: 10.7270/Q2GM89KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Mus musculus) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of PORCN in mouse Leydig cells overexpressing Wnt3A co-cultured with TM3 cells transfected with Wnt-luciferase gene after 24 hrs by lumine... | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Mus musculus) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of PORCN in mouse Leydig cells overexpressing Wnt3A co-cultured with TM3 cells transfected with Wnt-luciferase gene after 24 hrs by lumine... | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM374998 (N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(2'-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 676-80 (2016) Article DOI: 10.1021/acsmedchemlett.6b00038 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||