

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)c1ccc(Nc2nc3cc(CC(O)=O)c(C)cc3n2[C@@H]2C[C@H](C)CC(C)(C)C2)cc1
InChI Key: InChIKey=SVGIEKTYEVCEOB-FDDCHVKYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390647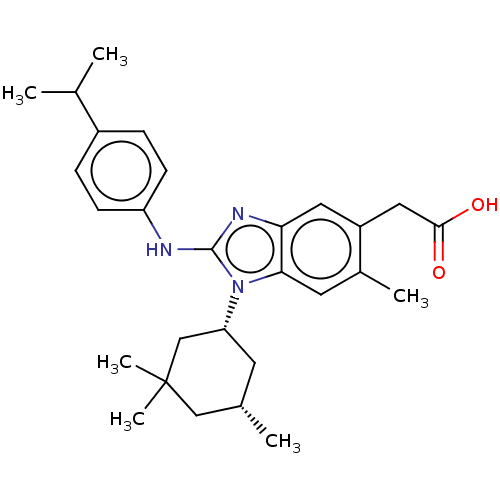 (US10442772, Example 2-237-1 | US9957235, 2-237 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390647 (US10442772, Example 2-237-1 | US9957235, 2-237 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390647 (US10442772, Example 2-237-1 | US9957235, 2-237 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390647 (US10442772, Example 2-237-1 | US9957235, 2-237 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390647 (US10442772, Example 2-237-1 | US9957235, 2-237 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||