

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM391338 Trans 2-(4-(3-Fluoroazetidin- 1-yl)-1-(3-((2-methyl-1,1- dioxido-2,3-dihydro- benzo[d]isothiazol-6- yl)amino)-4-oxo-4,5-dihydro- 1H-pyrazolo[4,3-c]pyridin-1- yl)cyclohexyl)acetonitrile::US9957264, Example 18-32
SMILES: CN1Cc2cc(Nc3nn(c4cc[nH]c(=O)c34)[C@@]3(CC#N)CC[C@@H](CC3)N3CC(F)C3)ccc2S1(=O)=O
InChI Key:
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM391338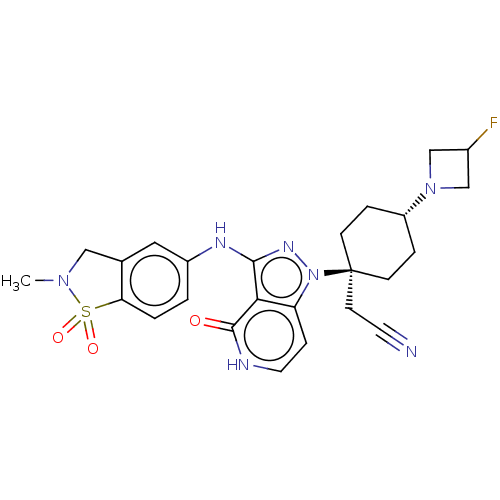 (Trans 2-(4-(3-Fluoroazetidin- 1-yl)-1-(3-((2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The ability of compounds to inhibit the activity of JAK1, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2KH0QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM391338 (Trans 2-(4-(3-Fluoroazetidin- 1-yl)-1-(3-((2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The ability of compounds to inhibit the activity of JAK1, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2KH0QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||