 Found 3 hits for monomerid = 392110
Found 3 hits for monomerid = 392110 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Isoform 2 of Nuclear receptor ROR-gamma (RORgT)
(Homo sapiens (Human)) | BDBM392110
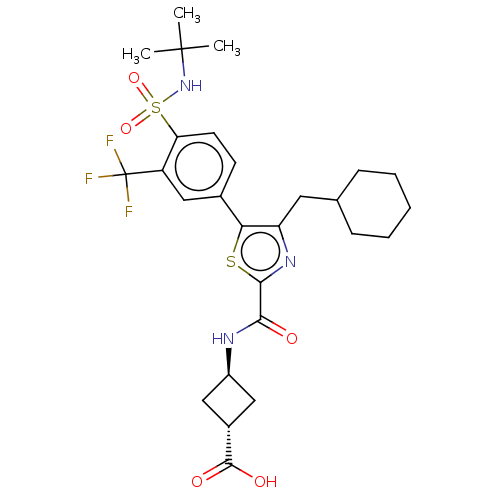
(US10301272, Example 9/2)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(cc1C(F)(F)F)-c1sc(nc1CC1CCCCC1)C(=O)N[C@H]1C[C@@H](C1)C(O)=O |r,wU:33.35,wD:35.40,(6.19,.46,;7.67,.06,;7.28,1.55,;8.76,1.15,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;2.92,-2.8,;4.43,-3.34,;4.83,-4.83,;5.22,-6.32,;6.36,-4.92,;3.34,-5.22,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-8.76,4.56,;-6.88,6.44,)| Show InChI InChI=1S/C27H34F3N3O5S2/c1-26(2,3)33-40(37,38)21-10-9-16(14-19(21)27(28,29)30)22-20(11-15-7-5-4-6-8-15)32-24(39-22)23(34)31-18-12-17(13-18)25(35)36/h9-10,14-15,17-18,33H,4-8,11-13H2,1-3H3,(H,31,34)(H,35,36)/t17-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Determination of a ligand mediated Gal4 promoter driven transactivation to quantify ligand binding to RORγ was performed as follows: DNA encodin... |
J Med Chem 52: 1814-27 (2009)
BindingDB Entry DOI: 10.7270/Q2S184TJ |
More data for this
Ligand-Target Pair | |
Isoform 2 of Nuclear receptor ROR-gamma (RORgT)
(Homo sapiens (Human)) | BDBM392110

(US10301272, Example 9/2)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(cc1C(F)(F)F)-c1sc(nc1CC1CCCCC1)C(=O)N[C@H]1C[C@@H](C1)C(O)=O |r,wU:33.35,wD:35.40,(6.19,.46,;7.67,.06,;7.28,1.55,;8.76,1.15,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;2.92,-2.8,;4.43,-3.34,;4.83,-4.83,;5.22,-6.32,;6.36,-4.92,;3.34,-5.22,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-8.76,4.56,;-6.88,6.44,)| Show InChI InChI=1S/C27H34F3N3O5S2/c1-26(2,3)33-40(37,38)21-10-9-16(14-19(21)27(28,29)30)22-20(11-15-7-5-4-6-8-15)32-24(39-22)23(34)31-18-12-17(13-18)25(35)36/h9-10,14-15,17-18,33H,4-8,11-13H2,1-3H3,(H,31,34)(H,35,36)/t17-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Cells were incubated for additional 16 h before renilla (REN) luciferase activities were measured sequentially in the same cell extract using a Dual-... |
J Med Chem 52: 1814-27 (2009)
BindingDB Entry DOI: 10.7270/Q2S184TJ |
More data for this
Ligand-Target Pair | |
Isoform 2 of Nuclear receptor ROR-gamma (RORgT)
(Homo sapiens (Human)) | BDBM392110

(US10301272, Example 9/2)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(cc1C(F)(F)F)-c1sc(nc1CC1CCCCC1)C(=O)N[C@H]1C[C@@H](C1)C(O)=O |r,wU:33.35,wD:35.40,(6.19,.46,;7.67,.06,;7.28,1.55,;8.76,1.15,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;2.92,-2.8,;4.43,-3.34,;4.83,-4.83,;5.22,-6.32,;6.36,-4.92,;3.34,-5.22,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-8.76,4.56,;-6.88,6.44,)| Show InChI InChI=1S/C27H34F3N3O5S2/c1-26(2,3)33-40(37,38)21-10-9-16(14-19(21)27(28,29)30)22-20(11-15-7-5-4-6-8-15)32-24(39-22)23(34)31-18-12-17(13-18)25(35)36/h9-10,14-15,17-18,33H,4-8,11-13H2,1-3H3,(H,31,34)(H,35,36)/t17-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
Cells were incubated for additional 16 h before firefly (FF) luciferase activities were measured sequentially in the same cell extract using a Dual-L... |
J Med Chem 52: 1814-27 (2009)
BindingDB Entry DOI: 10.7270/Q2S184TJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


