

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM397136 N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-[5-(phenylmethyl)-2-thiazolyl)]-3-pyridine carboxamide::US10456385, Example 29::US9987259, Example 29
SMILES: O=C(NC1CN2CCC1CC2)c1ccc(=O)n(c1)-c1ncc(Cc2ccccc2)s1
InChI Key: InChIKey=KGZOLQLWJGJBCT-UHFFFAOYSA-N
Data: 2 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor protein alpha-7 subunit (Homo sapiens (Human)) | BDBM397136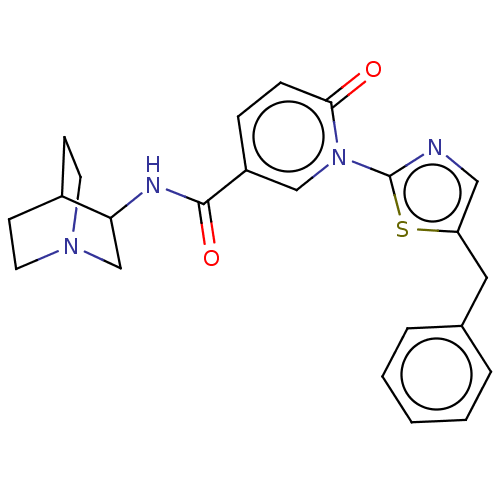 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-[5-(phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
SK BIOPHARMACEUTICALS CO., LTD. US Patent | Assay Description Activity of heteromeric α7 nAChR was measured via FlexStation-Ca2+ influx assay. In the present example, in consideration of α7 nAChR being... | US Patent US10456385 (2019) BindingDB Entry DOI: 10.7270/Q2V12762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor protein alpha-7 subunit (Homo sapiens (Human)) | BDBM397136 (N-(1-azabicyclo[2.2.2]octan-3-yl)-6-oxo-1-[5-(phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Yale University | Assay Description On the day of the assay, after removal of the growth media, the cells were washed once with an assay buffer (7 mM Tris-Cl, 20 mM HEPES, 20 mM NaCl, 5... | Bioorg Med Chem 17: 1764-71 (2009) BindingDB Entry DOI: 10.7270/Q2X92DNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||