 Found 9 hits for monomerid = 398043
Found 9 hits for monomerid = 398043 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
CFTR inhibitory factor, Cif
(Pseudomonas aeruginosa (strain UCBPP-PA14)) | BDBM398043
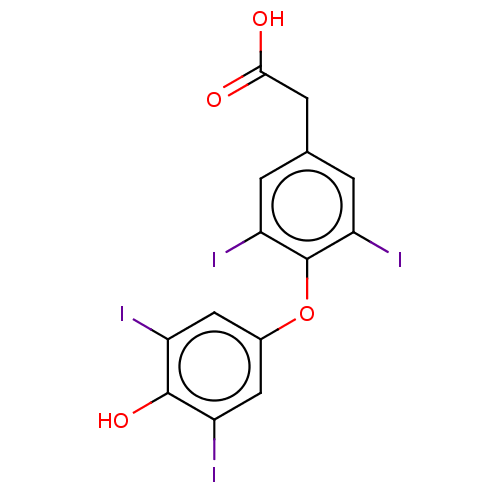
(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f... |
Bioorg Med Chem Lett 19: 4280-3 (2009)
BindingDB Entry DOI: 10.7270/Q2KW5JC9 |
More data for this
Ligand-Target Pair | |
ITGAV/ITGB3
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to purified integrin alphaVbeta3 receptor (unknown origin) incubated for 2 hrs |
J Med Chem 63: 7653-7662 (2020)
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human pFA-CMV fused PPARgamma expressed in HEK293T cells transfected with pFR-luciferase plasmid and pRL-SV40 plasmid... |
J Med Chem 63: 6727-6740 (2020)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant PPARgamma LBD (unknown origin) by isothermal titration calorimetry |
J Med Chem 63: 6727-6740 (2020)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha/gamma
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at GFP RXRalpha LBD (unknown origin) assessed as biotin-labeled SRC-1 recruitment incubated for 2 hrs by HT-FRET assay |
J Med Chem 63: 6727-6740 (2020)
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/gamma
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to RXRalpha LBD (unknown origin) by isothermal titration calorimetry |
J Med Chem 63: 6727-6740 (2020)
|
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at THRalpha (unknown origin) incubated for 14 to 16 hrs by dual glo luciferase reporter gene assay |
J Med Chem 63: 6727-6740 (2020)
|
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at THRbeta (unknown origin) incubated for 14 to 16 hrs by dual glo luciferase reporter gene assay |
J Med Chem 63: 6727-6740 (2020)
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/gamma
(Homo sapiens (Human)) | BDBM398043

(US10322118, Entry 1)Show InChI InChI=1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human pFA-CMV fused RXRalpha LBD expressed in HEK293T cells transfected with pFR-luciferase plasmid and pRL-SV40 plas... |
J Med Chem 63: 6727-6740 (2020)
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data