

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM398149 (S)-4-chloro-N-((3,3-difluoro-1-hydroxycyclohexyl)methyl)-1-(oxetan-3-yl)-1H-indole-3-carboxamide::US10323000, Compound 85::US10676433, Compound 85
SMILES: O[C@@]1(CNC(=O)c2cn(C3COC3)c3cccc(Cl)c23)CCCC(F)(F)C1
InChI Key: InChIKey=OIPFHTDSPCKTSD-SFHVURJKSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM398149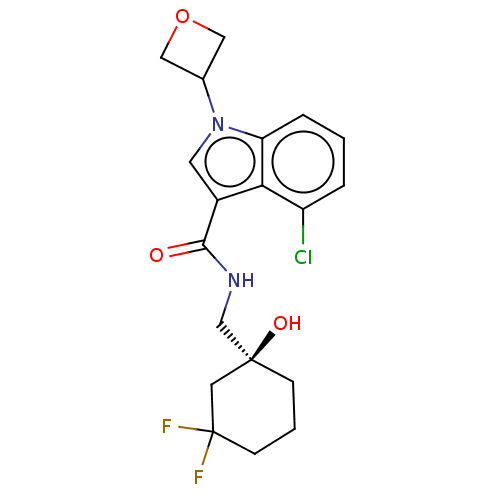 ((S)-4-chloro-N-((3,3-difluoro-1-hydroxycyclohexyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg | Assay Description Agonist-induced pore formation was determined by measuring cellular uptake of YO PRO fluorescence dye in HEK293 transfected with human P2X7 receptor.... | Bioorg Med Chem 16: 8574-86 (2008) BindingDB Entry DOI: 10.7270/Q26M395S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| hIL-1beta (Homo sapiens (Human)) | BDBM398149 ((S)-4-chloro-N-((3,3-difluoro-1-hydroxycyclohexyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description The activation of P2X7 by ATP leads to a fast transient activation of cells resulting in influx of Ca2+ followed by conversion of pro-IL-1β to a... | US Patent US10676433 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM398149 ((S)-4-chloro-N-((3,3-difluoro-1-hydroxycyclohexyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description Agonist-induced pore formation was determined by measuring cellular uptake of YO PRO fluorescence dye in HEK293 transfected with human P2X7 receptor.... | US Patent US10676433 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| hIL-1beta (Homo sapiens (Human)) | BDBM398149 ((S)-4-chloro-N-((3,3-difluoro-1-hydroxycyclohexyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg | Assay Description The activation of P2X7 by ATP leads to a fast transient activation of cells resulting in influx of Ca2+ followed by conversion of pro-IL-1β to a... | Bioorg Med Chem 16: 8574-86 (2008) BindingDB Entry DOI: 10.7270/Q26M395S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||